Formaldehyde (HCHO) is one of major pernicious indoor volatile organic compounds (VOCs) [1], which is mainly from the renovated rooms, furniture, clothes, cooking oil fume, etc. [2]. Long time exposure to HCHO can cause serious health problems [3], and HCHO is hard to be eliminated. Thus, great efforts to eliminate the indoor HCHO at ambient condition are urgently required. Generally, catalytic oxidation, including both photocatalysis [4] and thermal catalysis [5], is one of the most popular ways to eliminate HCHO among the widespread treatment techniques [6]. Some semiconductors, such as metal oxide (e.g., TiO2 [7]) and carbon nitride materials [8, 9] have been proved as promising photocatalysts. But the elimination efficiency is not satisfactory owing to that most of the catalysts can only be activated by UV irradiation [10], which takes only ~5% sunlight energy [11]. On the other hand, thermal catalytic oxidation, such as silver-ion exchanger nanocomposites [12], Pt/TiO2(110) [13] and noble metals/HZSM-5 [14], is also promising to remove VOCs, which demonstrates perfect catalytic efficiency. However, the relative high temperature limits the widespread applications of these metal oxides [15], such as Fe2O3, MnO2, NiO, Co3O4 and so on. Thus, it is important to find an appropriate material which can promote the HCHO oxidation at room temperature efficiently.
Graphene, as a single carbon layer two-dimensional crystal with honeycomb like lattice [16], has attracted remarkable attention due to its unique electronic structure [17], special intrinsic strength [18] and remarkable thermal conduction [19], making it applicable in various fields [20-22]. Importantly, it is one of the promising candidates for heterogeneous catalysts [23] owing to its huge surface-to-volume ratio. Single-atom catalyst (SAC), which was firstly proposed by Zhang et al. [24] in 2011, has been attracted intensive interest in recent years. SAC has been one of the new frontiers and hotspots in the field of catalysis. SACs possess higher catalytic oxidation activity [25-29] and excellent stability [26, 30-33]. Besides, the properties of graphene can be modified by doping [34]. Al has lager binding energy than that of Ag, Cu, Cd, Ir and Au on graphene, and it is much cheaper than Pt, Ti and Pd etc.[35]. Compared with other atoms, Al is much more active and can bind various gases, including VOCs and O2 [36]. Therefore, Al element is considered to dope into graphene for HCHO oxidation. Al-doped graphene is active for CO detection [36] and oxidation [37]. Importantly, Al-doped graphene can enhance HCHO molecules adsorption remarkably [38, 39]. Therefore, Al-doped graphene is expected as a promising material for HCHO catalytic oxidation.
In this work, first principles calculations are performed to explore the possibility of the catalytic oxidation of HCHO on Aldoped graphene, and the preferred pathway for the oxidation of HCHO is determined. The energy barriers of the oxidation process, charge transfer and the density of states (DOS) are also calculated to analyze the reaction mechanism.
Al-doped graphene with an Al atom replacing a C atom for different gases adsorption was reported by our previous works [1, 21, 40], which is also considered in this work for HCHO catalytic oxidation, the corresponding atomic structure is shown in Fig. S1a (Supporting information). The calculated binding energy between Al atom and graphene is -5.80 eV, which is much stronger than the cohesive energy of bulk Al (-3.39 eV). To further confirm the stability of single Al atom on the graphene, the mobility of the Al atom on graphene is also investigated through calculation the diffusion pathway of the Al atom from the site in initial state (IS) to its neighbor site in final state (FS). The diffusion energy barrier is 3.12 eV, as shown in Fig. S1b (Supporting information). It is known that the reaction could be difficult to take place at room temperature when the energy barrier is higher than 0.91 eV [41]. Such high barrier further confirms the stability of single Al atom on graphene at room temperature.
After understanding the stability of Al-doped graphene, HCHO oxidation process with the presence of O2 is then investigated. Therefore, the adsorption behavior of HCHO and O2 molecules on Al-doped graphene is calculated. After considering all the typical adsorption sites, the most stable configurations for HCHO and O2 adsorption are shown in Fig. S2 (Supporting information). It is clearly shown that the adsorbed O2 molecule is almost parallel with the graphene layer with two O—Al chemical bonds formation between the O2 molecule and Al doped graphene. The distance between the O2 and graphene layer is 1.71 Å, while the adsorption energy of O2 is -2.02 eV. Furthermore, the Mulliken charge calculation shows that O2 molecule donates 0.75 e charge to Al doped graphene after adsorption. More importantly, O=O bonding length increases from 1.23 Å to 1.44 Å. On the other hand, HCHO molecule is perpendicular to the plane of graphene and the O atom bonds with Al atom. The bond between HCHO and graphene layer is 1.86 Å. This agrees with the result of 1.88 Å reported in the literature [38]. The adsorption energy is -1.59 eV, while 0.18 e charge transfers from the graphene layer to the HCHO after adsorption. The C=O bond in HCHO increases from 1.22 Å to 1.28 Å.
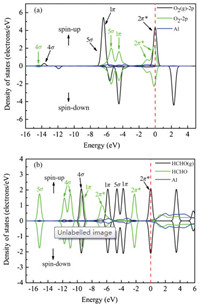
To further study the electronic structure, the partial density of states (PDOS) of the gaseous oxygen, the adsorbed oxygen and the Al atom were calculated and the results are shown in Fig. 1a. It is clear that the DOS of gaseous oxygen for spin-down and spin-up states are different, which agrees with other's result [42, 43]. Moreover, the peak of 2π* anti-bond orbital obviously shifts to lower energy level after adsorption. The similar phenomenon happens for 1p and 4s orbitals as well. And a new peak of 2π* antibond orbital appears in the left of the old one. The PDOS result confirms that O2 molecule accepts electrons from the graphene layer, which is consist with the above Mulliken charge calculation. Similarly, the PDOS of the gaseous HCHO, the adsorbed HCHO and the Al atom were shown in Fig. 1b. 2π* anti-bond orbital of gaseous HCHO molecule is half filled. After adsorption, 2π* anti-bond orbital shifts left and becomes full filled, which should be induced by the electrons transfer from the graphene layer to the HCHO molecule. The remarkable changes of PDOS of the gas molecules before and after adsorption indicate drastic interaction between the gas molecules and the Al doped graphene layer.
|
Download:
|
| Fig. 1. PDOS of (a) the isolated O2 molecule and the O2 adsorbed on Al doped graphene and (b) isolated HCHO molecule and the HCHO adsorbed on Al doped graphene with the corresponding orbitals are labelled in the top right corner. | |
From above results, it is clear that the O2 molecule is adsorbed in priority due to the stronger adsorption energy as well as high concentration in actual environment. Therefore, we firstly study the possibility of O2 dissociation or activation before HCHO adsorption, the corresponding dissociation pathway is shown in Fig. 2a with the structures of IS, transition state (TS), and FS shown in Fig. 2b. Based on the transition state search calculation, the energy barrier for O2 molecule dissociation on the graphene is 0.84 eV. It is normally considered that a reaction can go through at room temperature when the energy barrier is below 0.91 eV [1]. Therefore, we believe that O2 dissociation on Al-doped graphene can happen easily. In TS structure (Fig. 2), it is found that the O2 molecule adjusts the angle while O1 atom moves to the C atom layers with the O—O bond length increasing from 1.44 Å to 1.64 Å. At FS, O1 atom forms bond with a C atom in the Al doped graphene, and the bonded C atom also protrudes from the graphene layer due to the strong C—O chemical bond. After the O—O bond is broken, O2 atom only connects with the Al atom, leading to the enhanced Al-O bond energy with the bond length of Al-O1 decreasing from 1.87 Å to 1.80 Å and Al-O2 bond length decreasing from 1.84 Å to 1.68 Å. Meantime, the two Al-C bonds are weakened, which increase from 1.94 Å and 1.98 Å to 2.00 Å, respectively. Therefore, the result indicates that O2 can be dissociatively adsorbed on the Al doped graphene at ambient temperature.

|
Download:
|
| Fig. 2. (a) The dissociation reaction energy profile of the O2 molecule on Al doped graphene. (b) The structures of each state during the reaction. | |
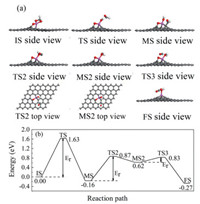
Based on literatures, it is known that there are two possible pathways for HCHO oxidation: (1) HCHO → CHO → CO → CO2 [44], (2) HCHO → HCOOH → CHO2 → CO2 [45]. Thus, both pathways for the HCHO catalytic oxidation on the Al doped graphene are considered. For the first pathway, the favorite adsorption configuration of HCHO molecule adsorbed on Al-doped graphene with O2 dissociatively adsorption is considered as the initial state (IS). As shown in Fig. 3a, it is found that in IS one of the three Al-C bonds is broken due to the formation of a new Al-O bond by the chemical attachment of the HCHO molecule. In addition, the C atom of HCHO binds with O2 atom with strong adsorption energy of -2.68 eV. The detailed oxidation process of HCHO calculated through linear synchronous transit/quadratic synchronous transit (LST/QST) and nudged elastic band (NEB) calculations and the corresponding structures of each reaction step are shown in Fig. 3. To better show the structures of transition state 2 (TS2) and intermediate state 2 (MS2), the corresponding top views are also shown. Starting from IS, it overcomes a barrier of 1.63 eV for the first H removal from HCHO in intermediate state (MS), where CHO and H atom connect with a C atom in the graphene layer and O1 atom, respectively. In the structure of TS as shown in Fig. 3a, HCHO is already dissociated and the H atom is chemically attached on O1 atom, but the CHO group is at highly active state without binding with any atom. Then the C—C bond between the CHO group and graphene is broken while the H atom from CHO is dissociated to form CO by a barrier of 1.03 eV. At the final step, O2 atom dissociates from Al doped graphene and bonds with CO to form CO2 molecule after overcoming an energy barrier of 0.21 eV. From above, we found that the first step is difficult to take place at room temperature because of its high energy barrier of 1.63 eV, thus we continue to explore the second pathway.
|
Download:
|
| Fig. 3. (a) The structures of each state during the oxidation pathway. (b) The first pathway of HCHO oxidation on Al doped graphene. | |
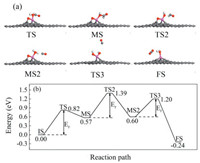
The second pathway is shown in Fig. 4 and IS is the same as that in the first pathway. At the first step, O atom from HCHO deviates from Al atom while one C— H bond in HCHO is broken and bonded with O1 atom to form HCOOH by consuming 0.82 eV. At next step, C-O bond in HCOOH is broken firstly while CHO floats above the graphene layer overcoming an energy barrier of 0.82 eV. Then H atom detaches from the CHO and bonds with O2, leading to the individual CO molecule. Finally, O1 atom leaves from Al to bond with CO to form CO2 molecule while H2O is also formed after a barrier of 0.60 eV. Based on the above calculations, the speed control step of the second pathway of HCHO oxidation is from IS to MS and from MS to MS2 with both barriers being 0.82 eV, which is lower than the critical barrier of 0.91 eV, indicating smooth reaction at ambient condition along the second pathway.
|
Download:
|
| Fig. 4. (a) The structures of each state during the oxidation pathway. (b) The second pathway of HCHO oxidation on Al doped graphene. | |
In order to further explore the possibility of HCHO oxidation in Al doped graphene by the two pathways at room temperature, we calculate the reaction time τ by the following equation (Eg. 1),

|
(1) |
where v = 1012 Hz, kB is the Boltzmann constant and it is equal to 8.63×10-5, T is the temperature (T = 300 K for room temperature). In the first pathway, Er = 1.63, 1.30 and 0.21 eV for the steps 1–3, respectively. Accordingly, the reaction time are τ1 = 2.20×1015 s at step 1, τ2 = 1.90×105 s at step 2 and τ3 = 3.33×10-9 s at step 3. Similarly, Er = 0.82, 0.82 and 0.60 eV for the steps 1–3 in the second pathway and the reaction time are obtained as τ1 = 56.9 s, τ2 = 56.9 s, τ3 = 1.16×10-2 s, respectively. Obviously, HCHO can be oxidized easily at 300 K along the second pathway, while the steps 1 and 2 in first pathway are difficult to carry out at 300 K.
In summary, the oxidation of HCHO molecule on Al doped graphene has been investigated by using density function theory (DFT) calculations. It is found that O2 molecules have priority to be adsorbed on the Al doped graphene. The process of O2 molecule dissociated into O atoms can take place at the room temperature with an energy barrier of 0.79 eV. For oxidation of HCHO, there are two possible pathways for HCHO oxidation to CO2 and H2O molecules. The first pathway (HCHO → CHO → CO → CO2) is difficult to occur at room temperature due to high barrier of 1.63 eV. However, the second pathway (HCHO → HCOOH → CO → CO2) can take place easily at ambient temperature with the highest energy barrier of 0.82 eV with reaction time 56.9 s at room temperature for the speed control step. Therefore, a novel and low-cost SAC system without any noble metal: Al doped graphene, was proposed for HCHO efficient oxidation and detoxification at room temperature. However, the desorption of H2O molecule at the final step is challenging, the adsorbed H2O molecule as oxidant to degrade formaldehyde will be considered in the future research.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 21777033, 21607029 and 41425015), Science and Technology Planning Project of Guangdong Province (No. 2017B020216003), and the Innovation Team Project of Guangdong Provincial Department of Education (No. 2017KCXTD012).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.12.023.
| [1] |
Q.G. Jiang, Z.M. Ao, S. Li, et al., RSC Adv. 4 (2014) 20290-20296. DOI:10.1039/C4RA01908C |
| [2] |
T. Salthammer, S. Mentese, R. Marutzky, Chem. Rev. 110 (2010) 2536-2572. DOI:10.1021/cr800399g |
| [3] |
M.C. Álvarez-Galván, V.A. de la Peña O'Shea, J.L.G. Fierro, et al., Catal. Commun. 4 (2003) 223-228. DOI:10.1016/S1566-7367(03)00037-2 |
| [4] |
B.H. Jing, Z.M. Ao, Z.Y. Teng, et al., Sustain. Mater. Technol. 16 (2018) 12-22. |
| [5] |
Z.X. Zhang, Z. Jiang, W.F. Shangguan, Catal. Today 264 (2016) 270-278. DOI:10.1016/j.cattod.2015.10.040 |
| [6] |
M. Kamal, S. Razzak, M. Hossain, Atmos. Environ. 140 (2016) 117-134. DOI:10.1016/j.atmosenv.2016.05.031 |
| [7] |
K. Nakata, T. Ochiai, T. Murakami, et al., Electrochim. Acta 84 (2008) 103-111. |
| [8] |
S.C. Yan, Z.S. Li, Z.G. Zou, Langmuir 25 (2009) 10397-10401. DOI:10.1021/la900923z |
| [9] |
Y. Su, W. Li, G. Li, et al., Chin. J. Catal. 40 (2019) 664-672. DOI:10.1016/S1872-2067(18)63201-2 |
| [10] |
R. Tejasvi, M. Sharma, K. Upadhyay, Chem. Eng. J. 262 (2015) 875-881. DOI:10.1016/j.cej.2014.10.040 |
| [11] |
G.A. Doschek, U. Feldman, Astrophys. J. 529 (2000) 599-604. DOI:10.1086/308244 |
| [12] |
E. Sakardina, T. Kravchenko, A. Kalinichev, et al., Phys. Chem. 464 (2015) 202-205. |
| [13] |
M.Z. Jing, W.Y. Song, L.L. Chen, et al., J. Phys. Chem. C 122 (2017) 438-448. |
| [14] |
A.V. Kucherova, I.M. Sineva, S. Ojalab,et al., Adsorptive-catalytic removal of CH3OH, CH3SH, and CH3SSCH3 from air over the bifunctional system noble metals/HZSM-5, in: R. Xu, Z. Gao, J. Chen (Eds.), Studies in Surface Science and Catalysis, Amsterdam, 2007, pp. 1129-1136.
|
| [15] |
C. Ma, D. Wang, W. Xue, et al., Environ. Sci. Technol. 45 (2011) 3628-3634. DOI:10.1021/es104146v |
| [16] |
A.C. Ferrari, J.C. Meyer, V. Scardaci, et al., Phys. Rev. Lett. 97 (2006) 187401. DOI:10.1103/PhysRevLett.97.187401 |
| [17] |
O. Leenaerts, B. Partoens, F.M. Peeters, Phys. Rev. B 79 (2009) 235-440. |
| [18] |
C. Lee, X. Wei, J.W. Kysar, et al., Science 321 (2008) 385-388. DOI:10.1126/science.1157996 |
| [19] |
A. Balandin, S. Ghosh, W. Bao, et al., Nano Lett. 8 (2008) 902-907. DOI:10.1021/nl0731872 |
| [20] |
L. Saghatforoush, M. Hasanzadeh, N. Shadjou, Chin. Chem. Lett. 25 (2014) 655-658. DOI:10.1016/j.cclet.2014.01.014 |
| [21] |
Q.G. Jiang, Z.M. Ao, Q. Jiang, Phys. Chem. Chem. Phys. 15 (2013) 10859-10865. DOI:10.1039/c3cp00128h |
| [22] |
Q.G. Jiang, Z.M. Ao, D.W. Chu, et al., J. Phys. Chem. C 116 (2012) 19321-19326. DOI:10.1021/jp3050466 |
| [23] |
J. Lu, C. Aydin, N.D. Browning, et al., Angew. Chem. Int. Ed. 51 (2012) 5842-5846. DOI:10.1002/anie.201107391 |
| [24] |
Q.B. Tao, W.A. Qin, L.J. Yue, et al., Nat. Chem. 3 (2011) 634-641. DOI:10.1038/nchem.1095 |
| [25] |
W. Tang, Z. Hu, M. Wang, et al., J. Catal. 273 (2010) 125-137. DOI:10.1016/j.jcat.2010.05.005 |
| [26] |
D. Deng, X. Chen, L. Yu, et al., Sci. Adv. 1 (2015) e1500462. DOI:10.1126/sciadv.1500462 |
| [27] |
Z. Huang, X. Gu, Q.Q. Cao, et al., Angew. Chem. Int. Ed. 51 (2012) 4198-4203. DOI:10.1002/anie.201109065 |
| [28] |
C.B. Zhang, F.D. Liu, Y.P. Zhai, et al., Angew. Chem. Int. Ed. 51 (2012) 9628-9632. DOI:10.1002/anie.201202034 |
| [29] |
Y. Qu, B. Chen, Z. Li, et al., J. Am. Chem. Soc. 141 (2019) 4505-4509. DOI:10.1021/jacs.8b09834 |
| [30] |
J.Y. Liu, ACS Catal. 7 (2016) 34-59. |
| [31] |
W.J. Yang, Z.Y. Gao, X.S. Liu, et al., Fuel 243 (2019) 262-270. DOI:10.1016/j.fuel.2019.01.125 |
| [32] |
J. Yuan, W.H. Zhang, X.X. Li, et al., Chem. Commun. 54 (2018) 2284-2287. DOI:10.1039/C7CC08713F |
| [33] |
C. Tian, H. Zhang, X. Zhu, et al., Appl. Catal. B 261 (2020) 118178. DOI:10.1016/j.apcatb.2019.118178 |
| [34] |
A.K. Geim, Science 324 (2009) 1530-1534. DOI:10.1126/science.1158877 |
| [35] |
C. Gong, G. Lee, B. Shan, et al., J. Appl. Phys. 108 (2010) 123711. DOI:10.1063/1.3524232 |
| [36] |
J.Y. Dai, J.M. Yuan, P. Giannozzi, Appl. Phys. Lett. 95 (2009) 232105. DOI:10.1063/1.3272008 |
| [37] |
G. Wang, J. Su, Y. Gong, et al., Angew. Chem. Int. Ed. 49 (2010) 1302-1305. DOI:10.1002/anie.200906473 |
| [38] |
M. Chi, Y.P. Zhao, Comput. Mater. Sci. 46 (2009) 1085-1090. DOI:10.1016/j.commatsci.2009.05.017 |
| [39] |
X. Qin, Q.Y. Meng, W. Zhao, Surf. Sci. 605 (2011) 930-933. DOI:10.1016/j.susc.2011.02.006 |
| [40] |
Z.M. Ao, Q. Jiang, R.Q. Zhang, et al., J. Appl. Phys. 105 (2009) 074307. DOI:10.1063/1.3103327 |
| [41] |
D.C. Young, Computational Chemistry:A Practical Guide for Applying Techniques to Real World Problems. New York: John Wiley & Sons Inc., 2001.
|
| [42] |
C. Cheng, X.L. Zhang, M.Y. Wang, et al., Phys. Chem. Chem. Phys. 20 (2018) 3504-3513. DOI:10.1039/C7CP07161B |
| [43] |
Q.G. Jiang, J.F. Zhang, Z.M. Ao, et al., Front. Chem. 6 (2018) 187. DOI:10.3389/fchem.2018.00187 |
| [44] |
Y.L. Cao, Z.X. Chen, Phys. Chem. Chem. Phys. 9 (2007) 739-746. DOI:10.1039/B610691A |
| [45] |
M. Nolan, S.C. Parker, G.W. Watson, Surf. Sci. 595 (2005) 223-232. DOI:10.1016/j.susc.2005.08.015 |
 2020, Vol. 31
2020, Vol. 31 

