b Sino-Singapore International Joint Research Institute, Sino-Singapore Guangzhou Knowledge City, Guangzhou 510006, China;
c Environmental Engineering Program, Department of Civil Engineering, Auburn University, Auburn AL 36849, United States
Chromate is an important chemical material of industries such as chemical industry, electroplating, metallurgy and dyeing [1-3]. The manufacture of chromates produces a great amount of chromite ore processing residue (COPR). In China, millions of tons of COPR are discharged in annually [4]. These residues contain highly soluble, mobile and carcinogenic toxic Cr(VI) [5], posing a great threat to soil and groundwater [6, 7]. The traditional treatment of COPR is immobilization, transforming Cr(VI) into Cr(III) by reducing agent and landfill [8]. Although the reduction treatment can effectively reduce the toxicity of COPR, the reduction product is unstable and will be reoxidized to Cr(VI) under the influence of oxygen and manganese oxide [9]. At the same time, it also take up a lot of land resources and causes waste of chromium. Hence, the development of an effective Cr(VI) extraction method, which can achieve both COPR detoxification and chromium recovery, is of great significance for COPR treatment.
Recently, several feasible methods for extracting Cr(VI) from COPR have been reported, such as acid leaching [10, 11], salt solution leaching [12] and roasting-leaching [13, 14]. These methods have high Cr(VI) extraction efficiencies, but can only leach the acid/water-leachable Cr(VI). A large amount of Cr(VI) is still structurally incorporated in the mineral phases, so the residue cannot be detoxified. Previous studies of our team have shown that effectively controlling phase transformation of minerals can extract heavy metals from residues. Lv et al. extracted Cr(VI) from chromium-containing Mg-Al layered double hydroxide (LDH) by controlling the transformation of Cr(VI)-LDH to HCO3-LDH [15]. Liu et al. recovered Pb from Pb2+ absorbed Mg(OH)2 via CO2-assisted phase transformation [16]. Liu et al. extracted Cr(VI) effectively from hazardous gypsum by controlling the transformation of mineral phase and chromium species [17]. However, in complex system such as CORP, there is still a lack of research into the Cr(VI) existing states and mineral phases transformation.
COPR is a typical example of a complicated cementitious system, containing many crystalline and amorphous phases with extensive variability in chemical composition, where Cr(VI) can be structurally incorporated in the minerals [18]. Some literatures reveal that Cr(VI) is closely related to the mineral phases of hydrogarnet, hydrocalumite, ettringite and hydrotalcite [18-20]. Our previous studies have shown that mineral phase transforma-tion can be controlled under conditions of mineralizer and certain temperature [21, 22]. Therefore, it is feasible to focus on Cr(VI)-loaded mineral phase and extract Cr(VI) from COPR by minerali-zation and thermal treatment.
The purpose of this work is based on investigation of Cr(VI) contained mineral phases, the complete detoxification and Cr(VI) recovery of CORP from a microscale perspective, followed by optimization of mineralizer species and treatment conditions according to Cr(VI) extraction efficiency and leaching toxicity (TCLP). Moreover, relevant mechanism for separation or desorp-tion of Cr(VI) from CORP was precisely elucidated by multiple analytical methods and finally the stability of detoxified residue was proved by stir-flow experiment.
The initial COPR was dark brown with a moisture content of 7.1%, and the pH of the mixture of 1 g sample and 10 mL water was greater than 14. Elements content of the COPR sample characterized by inductively coupled plasma optical emission spectrometer (ICP-OES) was shown in Table 1, with the highest Fe content of 182.62 g/kg and a total Cr of 71.62 g/kg. The TCLP test [23] showed that the Cr(VI) concentration in leachate was 430 mg/L, which was much higher than the identification standards for hazardous wastes of China (GB 5085.6-2007, 5 mg/L), revealing that the COPR has a significant threat to the environment. X-ray photoelectron spectroscopy (XPS) was used to analyze the valence information of Cr in COPR. Two pronounced peaks were observed in the XPS spectra (Cr 2p) of the sample (Fig. 1a), 579.0 eV and 576.5 eV respectively, which confirmed the co-existence of Cr(VI) and Cr(III) in the sample. The concentration of Cr(VI) in initial COPR analyzed by alkali digestion method, was 14, 100 mg/kg, and the Cr(III) concentration was measured to be 57, 520 mg/kg.
|
|
Table 1 Elements concentration of initial COPR (ICP-OES). |

|
Download:
|
| Fig. 1. (a) XPS spectra and (b) XRD pattern of the COPR sample. | |
Four mineral phases (spinel, hydrocalumite, calcite and melilite) can be identified directly from X-ray diffraction (XRD) pattern of initial COPR (Fig. 1b). Spinel is a raw material for chromate industry, which consists of Fe3O4 and Mg(FeCrAl)2O4 and had not been fully reacted during the roasting process. Melilite is a common rock phase that is easily doped in the raw materials and is likely to occur in COPR [24]. Calcite and hydrocalumite are considered to be weathering products during landfill process [25, 26]. There is a sodium-containing hydrocalumite for the addition of sodium carbonate in productive process, and Cr(VI) is considered to be structurally incorporated in this mineral phase [20].
To reveal the relationship between Cr(VI) and minerals, A field emission scanning electron microscope with energy dispersive spectrometer (FESEM-EDS) was used for further analysis. Depending on the image, the sample has various surface morphologies and structures (Fig. 2a). The particles can be roughly divided into four categories: Stacked sheet, agglomerate, rod, and cube (Figs. 2b-e). FESEM-EDS analysis showed that the atom ratio of Na, Ca, Al, O of the stacked sheet were 1:6.5:2.9:16 (Fig. S2 in Supporting information) corresponding to the hydrocalumite [NaCa4Al2O6 (SO4)1.5 15H2O] in XRD pattern, while the atom ratio of Mg, AL, Fe, Cr, O of the agglomerate were 1:0.6:1.5:0.4:6 (Fig. S3 in Supporting information) corresponding to spinel [Mg(FeCrAl)2O4]. FESEM-EDS analysis data indicated that these two minerals have an obvious chromium signal. Since Cr in chromite mainly presents in the form of Cr(III), Cr(VI) was considered to be mainly present in hydro-calumite [NaCa4Al2O6(SO4/CrO4)1.5 15H2O].

|
Download:
|
| Fig. 2. SEM images of COPR (a), stacked sheet (b), agglomerate (c), rod (d), and cube (e). | |
In the FESEM-EDS analysis, the Ca, C, O atomic ratio of cubic particles was 1:2:6.4 (Fig. S4 in Supporting information), while the Ca, Al, Si, O ratio of rod-shaped particles was 1:3:3:11 (Fig. S5 in Supporting information), corresponding to calcite (CaCO3) and melilite (CaAl2Si2O8) respectively. Besides, there was a feeble signal of Cr in these minerals. Considering the leaching process of chromate manufacturing, tiny amount of Cr(VI) exists on the surface of these two minerals.
A series of experiments with various mineralizers were carried out for COPR treatment. Mineralizers include different concentrations of inorganic acid and alkali solution, and water was used as a control experiment. The temperature was maintained at 120 ℃ for 2 h. After hydrothermal mineralization treatment, the concentration of Cr(VI) in supernatants and leachates of solids (TCLP method) were measured (Fig. 3a). It was easy to find that 1 mol/L Na2CO3 solution was an ideal mineralizer with the highest concentration in the supernatant and the lowest in the leachate.

|
Download:
|
| Fig. 3. (a) Cr(VI) concentrations in supernatant and leachate of optimized experiment; (b)TCLP of residues of various heating time. | |
1 mol/L Na2CO3 solution was used for further study to optimize the hydrothermal temperature and heating time. Experiments were carried out at a temperature of 100-180 ℃ (heating for 2 h). The TCLP test results of the treated residues were shown in Fig. S6 (Supporting information). Higher temperatures were conducive to detoxification. The residues met the treatment standard at a temperature of 120 C, and achieved the desired treatment effects at temperatures above 150 C. Experiments with different heating times were carried out at 120 ℃. As showed in Fig. 3b, after 3 h hydrothermal mineralization the TCLP of residue satisfied treat-ment standard (HJ/T 301-2007, total Cr < 9 mg/L, Cr(VI) < 3 mg/L). In order to reduce energy consumption and save time, we optimized the treatment conditions of 1 mol/L Na2CO3 solution and heated at 120 ℃ for 3 h. Under the optimal treatment conditions, 95% Cr(VI) in COPR was removed, and the Cr(VI) concentration of TCLP was decreased to 1.6 mg/L.
To reveal the mineral phases transformation in COPR during hydrothermal mineralization treatment, residues of different heating time (20-720 min) were obtained in a 1 mol/L Na2CO3 solution and at 120 C. Fig. 4a showed the XRD pattern of this process, the diffraction peak of hydrocalumite disappeared rapidly in the initial stage of the reaction, and Cr(VI) was transferred to the supernatant, but the Cr(VI) in the residue remained highly toxic at this time according to Fig. 3b. Along with the prolongation of hydrothermal treatment, the diffraction peaks of calcite increased significantly, as the calcite crystals grew, the toxicity of Cr(VI) in the residue was further decreased.

|
Download:
|
| Fig. 4. XRD pattern of treatment process (a) and SEM images of 3 h (b), 6 h (c), 12 h (d), 24 h (e) heating. | |
The SEM images of this process were shown in Figs. 4b-e, cubic particles with large size and complete surface appeared gradually. During hydrothermal mineralization, mineralizers reacted with chromium-contained/absorbed hydrocalumite and calcite (reac-tion 1 and 2), the structure of hydrocalumite was disintegrated and the Cr(VI) was released. At the same time, with the growth of calcite crystal, the adsorbed Cr(VI) on the surface of calcite was removed, and the residue was detoxified eventually. The reaction mechanism diagram was shown in Scheme 1.
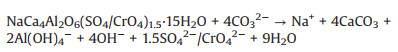
|
(1) |
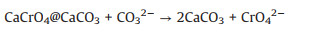
|
(2) |

|
Download:
|
| Scheme 1. Mechanism diagram of COPR detoxification. | |
In the study of environmental chemistry, stir-flow methods are used to simulate the release kinetics of ion exchange and adsorption-desorption processes in the field [27]. This experiment simulated the release of chromium from residue in natural environment, where PIPPS buffer was added to simulate the pH conditions of soil, and NaNO3 was added to maintain the ionic strength of the system [28]. Fig. 5 demonstrated the variation of pH and the concentration of total Cr during the stir-flow experiment between initial and treated residues. Throughout the leaching process, the system pH of initial sample was maintained in range of 9-11 due to the alkaline substances in CORP, while the system pH of treated residue rapidly changed from weak alkaline to weak acidic. Both samples had a total Cr release peak at the beginning of leaching, 200 mg/L and 5 mg/L respectively, and then rapidly decreased to a much lower and stable concentration of approximately, 0.9 mg/L. After hydrothermal mineralization, due to the disappearance of hydrocalumite and growth of calcite, the emission of chromium to the environment can be greatly reduced. However, there was still a small amount of leachable Cr(VI) in the treated residue, which was supposed to be related to another potential Cr(VI)-containing mineral phase, calcite, where Cr(VI) was doped [29].

|
Download:
|
| Fig. 5. Total Cr concentrations and pH value of stir-flow effluents of initial (a) and treated (b) samples. | |
In summary, Cr(VI) in the lime-free roasting COPR was mainly structurally incorporated in the structure of hydrocalumite, and a small amount of Cr(VI) could also be adsorbed on the surface of other mineral phases (e.g., calcite and melilite). The ideal mineralizer for detoxification and Cr(VI) extraction of COPR is Na2CO3. During the treatment, Cr(VI) was released to supernatant as the structure of hydrocalumite collapsed and calcite crystal grew. After the optimized hydrothermal mineralization treatment with 1 mol/L Na2CO3 solution and heated at 120 ℃ for 2 h, 95% Cr(VI) was removed and the toxicity of residue reached the treatment standard (HJ/T 301-2007, total Cr < 9 mg/L, Cr(VI) < 3 mg/L). This is a clean method for COPR detoxification without secondary pollution. In this process, the mineralizer Na2CO3 which is a raw materials for chromate production and chromium remaining in supernatant can be directly reused for chromate production. Hence, no other pollutants are introduced into the treatment process.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (No. 21836002), the Young Innovative Talents Project in Higher Education of Guangdong (No. 2018KQNCX002), Guangdong Innovative and Entrepreneurial Research Team Program (No. 2016ZT06N569), the Fundamental Research Funds for the Central Universities (No. D2192000), the Shaoguan Special Fund for Soil Pollution Prevention and Control (No. 2017sgtyfz103) and the Youth Talent Promotion Project of Guangzhou Science and Technology Association (No. X20200301029).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.040.
| [1] |
E.L.J. Kleynhans, B.W. Neizel, J.P. Beukes, P.G. van Zyl, Miner. Eng. 92 (2016) 114-124. DOI:10.1016/j.mineng.2016.03.005 |
| [2] |
K. Matern, H. Weigand, A. Singh, T. Mansfeldt, Environ. Sci. Pollut. Res. 24 (2017) 3582-3592. DOI:10.1007/s11356-016-8110-2 |
| [3] |
Q. Zhao, C. Liu, D. Yang, et al., Process Saf. Environ. Prot. 105 (2017) 91-100. DOI:10.1016/j.psep.2016.09.017 |
| [4] |
W. Liu, J. Zheng, Z. Wu, Z. Liu, Z. Lin, Prog. Chem. 29 (2017) 1053-1061. |
| [5] |
Environmental Protection Agency, Integrated Risk Information System (IRIS), Reference Dose for Oral Exposure to Chromium(III) and Chromium(VI), Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, EPA, Cincinnati, 1988.
|
| [6] |
E. Tassi, M. Grifoni, F. Bardelli, et al., Environ. Sci. Process. Impacts 20 (2018) 153-163. |
| [7] |
I. Aharchaou, J.S. Py, S. Cambier, et al., Environ. Toxicol. Chem. 37 (2018) 983-992. DOI:10.1002/etc.4044 |
| [8] |
Q. Zhang, G. Chang, T. Gao, et al., Review of chromium residue and chromiumcontaining waste water treatment, 20176th International Conference on Energy and Environmental Protection (ICEEP 2017), Atlantis Press, 2017.
|
| [9] |
A.D. Apte, S. Verma, V. Tare, P. Bose, J. Hazard. Mater. 121 (2005) 215-222. DOI:10.1016/j.jhazmat.2005.02.010 |
| [10] |
J.M. Tinjum, C.J. Benson, T.B. Edilb, Sci. Total Environ. 391 (2008) 13-25. DOI:10.1016/j.scitotenv.2007.10.041 |
| [11] |
Q. Zhao, C. Liu, B. Li, et al., Process Saf. Environ. Prot. 113 (2018) 78-87. DOI:10.1016/j.psep.2017.10.002 |
| [12] |
W. Liu, X. Xu, Y. Wang, et al., Chin. Sci. Bull. 55 (2010) 373-377. DOI:10.1007/s11434-009-0716-z |
| [13] |
K.J. Sreeram, T. Ramasami, J. Environ. Monit. 3 (2001) 526-530. DOI:10.1039/b104303j |
| [14] |
M.P. Antony, V.D. Tathavadkar, C.C. Calvert, A. Jha, Metall. Mater. Trans. B 32 (2001) 987-995. DOI:10.1007/s11663-001-0087-6 |
| [15] |
X. Lv, Z. Chen, Y. Wang, F. Huang, Z. Lin, ACS Appl. Mater. Interfaces 5 (2013) 11271-11275. DOI:10.1021/am4035009 |
| [16] |
X. Liu, K. Song, W. Liu, et al., Environ. Sci. Nano 6 (2019) 467-477. DOI:10.1039/C8EN01173G |
| [17] |
W. Liu, J. Zheng, X. Ou, et al., Environ. Sci. Technol. 52 (2018) 13336-13342. DOI:10.1021/acs.est.8b02213 |
| [18] |
S. Hillier, M.J. Roe, J.S. Geelhoed, A.R. Fraser, J.G. Farmer, E. Paterson, Sci. Total Environ. 308 (2003) 195-210. DOI:10.1016/S0048-9697(02)00680-0 |
| [19] |
C.T. Mills, C.R. Bern, R.E. Wolf, et al., Environ. Sci. Technol. 51 (2017) 11235-11243. DOI:10.1021/acs.est.7b01719 |
| [20] |
M. Chrysochoou, D. Dermatas, J. Hazard. Mater. 141 (2007) 370-377. DOI:10.1016/j.jhazmat.2006.05.081 |
| [21] |
W. Liu, F. Huang, Y. Liao, et al., Angew. Chem. Int. Ed. 47 (2008) 5619-5622. DOI:10.1002/anie.200800172 |
| [22] |
W. Xu, X. Li, Q. Zhou, et al., Process Saf. Environ. Prot. 89 (2011) 179-185. DOI:10.1016/j.psep.2010.11.002 |
| [23] |
NEPA, China, Solid Waste-extraction Procedure for Leaching Toxicity-Sulphuric Acid and Nitric Acid Method (HJ/T299-2007), Environmental Press, Beijing, 2007.
|
| [24] |
Q. Zhao, C. Liu, D. Yang, et al., Process Saf. Environ. Prot. 105 (2017) 91-100. DOI:10.1016/j.psep.2016.09.017 |
| [25] |
F. Corinna, D. Reiner, M. Katrin, T. Mansfeldt, J. Soils Sediments 13 (2013) 1170-1179. DOI:10.1007/s11368-013-0714-2 |
| [26] |
T. Boecher, J.M. Tinjum, H. Xu, J. Residuals Sci. Technol. 9 (2012) 131-141. |
| [27] |
J.E. Lopez-Periago, M. Arias-Estevez, J.C. Novoa-Munoz, et al., Soil Sci. Soc. Am. J. 72 (2008) 63-72. DOI:10.2136/sssaj2006.0079 |
| [28] |
M. Zhang, C. Yang, M. Zhao, et al., J. Hazard. Mater. 342 (2018) 242-251. DOI:10.1016/j.jhazmat.2017.07.039 |
| [29] |
N. Sanchezpastor, A.M. Gigler, J.A. Cruz, et al., Cryst. Growth Des. 11 (2011) 3081-3089. DOI:10.1021/cg200357c |
 2020, Vol. 31
2020, Vol. 31 


