b International Center of Future Science, Jilin University, Changchun 130012, China
Zeolites are a family of microporous crystalline materials that have been widely applied in many chemical industries and sustainable processes as adsorbents, molecular sieves, and catalysts [1-6]. To synthesize a specific zeolite framework, organic structure-directing agents (OSDAs), which are usually organic ammonium or phosphonium cations, are added into the reaction system [7, 8]. These organic cations interact with inorganic species via non-bonding host-guest interactions and lead to the formation of specific zeolite frameworks. By selecting OSDAs with different geometrical and chemical properties, a large number of zeolite frameworks have been synthesized during the past decades [9, 10].
Many experimental efforts have been made to understand the complicated host-guest interactions between OSDAs and zeolite frameworks [11, 12], but the exact mechanisms for the crystallization of zeolites remain unclear. Molecular simulations provide an alternative way to understand the host-guest interactions between OSDAs and zeolite frameworks. In particular, molecular simulations may reveal the most probable locations and conformations of OSDAs within the pores of zeolites [13-15]. On the other hand, molecular simulations may provide useful information about the host-guest interactions from the energetic point of view, shedding light on the role of OSDAs during the formation of zeolites [16-21].
STW is a well-known chiral zeolite framework type with helical 10-ring channels formed by its featured stw cavities ([465882102]) and d4r cages [22]. The stw cavities are stacked along the [001] direction by sharing their 10-ring windows, and the d4r cages are embedded between adjacent stw cavities (Fig. S1 in Supporting information). Because of their potential applications in enantio-selective catalysis and separation, many STW-type zeolites with different framework compositions have been synthesized using various types of OSDAs. For instance, Zou and co-workers synthesized the first STW-type zeolite, silicogermanate SU-32, by using diisopropylamine as the OSDA in 2008 [23]; Camblor and co-workers synthesized pure-silica zeolite HPM-1 by using 2-ethyl-1, 3, 4-trimethylimidazolium cations as the OSDA in 2012, which was the first pure-silica polymorph of STW [24]; Shi and co-workers utilized 2-ethyl-1, 3, 4-trimethylimidazolium as the OSDA to synthesize boron silicate STW and N, N-diethylethylenediamine to synthesize germanosilicate STW [25, 26]; Davis and co-workers utilized chiral imidazolium-based dications to synthesize enan-tiomerically enriched STW, which exhibited enantioselectivity for the ring-opening reaction of epoxides and enantioselective adsorption of 2-butanol [27]; Hong and co-workers produced pure-silica STW zeolites using a series of imidazolium derivatives and 1, 2, 3, 5-tetramethylpyrazolium [28]; Camblor and co-workers utilized achiral dimethylated imidazolium-based dications with linkers of four, five and six methylenes as OSDAs to synthesize enantiopure STW recently [29].
To facilitate the discovery of new STW-type zeolites, computer simulations have been used to study the structure-directing effect of different OSDAs. For instance, Davis and co-workers predicted three imidazolium-based cations as OSDAs using molecular simulations and successfully synthesized pure-silica STW zeolites in experiment using the predicted OSDAs [30]; Camblor and co-workers conducted DFT calculations to study the structure-direction effects of three dimethylated imidazolium-based dications with linkers of four, five, and six methylenes toward the formation of STW [29]; Deem and co-workers predicted a large number of chiral imidazolium dimers using genetic algorithm for the synthesis of pure-silica chiral STW [31]. In general, alkylated imidazolium and pyrazolium cations exhibit strong interactions toward the framework of STW, which are promising candidate OSDAs for the formation of STW-type zeolites. However, to the best of our knowledge, there is no systematic report on the structure-directing effect of this important OSDA family.
In this study, we use molecular simulation techniques to investigate the non-bonding host-guest interactions between the framework of STW and 21 alkylated imidazolium and pyrazolium cations, including 9 successful OSDAs that have directed the formation of STW-type zeolites in experiment and 12 unsuccessful OSDAs (Fig. 1). Through these calculations, we want to find the most important distinctions in molecular structures between successful and unsuccessful OSDAs, which will provide important guidance to the design of new OSDAs and the rational synthesis of specific zeolitic frameworks.

|
Download:
|
| Fig. 1. 21 experimental OSDAs and their calculated non-bonding host-guest interaction energies with the framework of STW (kcal (mol OSDA) -1). Successful and unsuccessful OSDAs for STW synthesis are shown in green and red, respectively. | |
All calculations were performed using BIOVIA Materials Studio package [32]. The initial structure model of STW (space group P6122) was taken from literature [25] and refined as a pure-silica polymorph using the CVFF force field [33]. The 21 alkylated imidazolium and pyrazolium OSDAs involved in this study can be divided into three groups, including monoquaternary imidazo-lium-based cations (Group A), monoquaternary pyrazolium-based cations (Group B), and diquaternary imidazolium-based cations (Group C). Every OSDA cation was first optimized by DFT calculations with the generalized gradient approximation and the PBE functional [34]. Mülliken atomic charges and the dipole moments were calculated after DFT optimization [35]. Then, OSDA cations were manually put into the helical channels of STW. We tried several locations and orientations for each OSDA cation and kept the ones giving the best total energies after further geometry optimization. Finally, we performed simulated annealing molecu-lar dynamics simulations. The annealing temperature ranged from 300 K to 1000 K with an increment of 10 K. 1.0 fs of molecular dynamic simulations were performed in every heating-cooling cycle, and every cycle was repeated for 10 times. The locations and conformations of all OSDAs were further optimized at the end of each cycle. For each OSDA, we chose the cycle giving the best total energy to calculate the host-guest interaction energy Einter, according to the following formula:
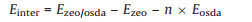
|
where Ezeo/osda is the total energy of zeolite framework together with n OSDA cations added in its channels, Ezeo is the energy of zeolite framework STW, and Eosda is the energy of a free OSDA cation.
All atoms in STW frameworks were kept fixed throughout the calculations. Van der Waals and electrostatic interactions were calculated by the atom-based and Ewald summation methods, respectively. The CVFF force field was used to describe the host-guest interactions, which has been well accepted for the simulation of zeolites and related materials [33]. The positive charges of the OSDA cations were compensated using the uniform charge background method, where atomic charges for framework oxygen atoms were fixed to -1.2 e and the charges for framework silicon atoms were reduced from +2.4 e until charge neutrality was achieved [36].
The 9 OSDAs that could direct the formation of STW in experiment are shown in green in Fig. 1, including four monoquaternary imidazolium-based cations (Group A: OSDAs 1 to 4), one monoquaternary pyrazolium-based cation (Group B: OSDA 12) and four diquaternary imidazolium-based cations (Group C: OSDAs 18 to 21). Their non-bonding host-guest interaction energies with STW framework are shown in brackets in Fig. 1. All successful OSDAs exhibit stronger non-bonding interactions with STW framework than unsuccessful ones in the same group, indicating stronger structural-directing effect toward STW. This agrees well with the experimental results.
Group A consists of 11 monoquaternary imidazolium-based cations (OSDAs 1 to 11). The strongest host-guest interactions occur when each stw cavity is occupied by one OSDA cation (Fig. 2A and Fig. S1). OSDAs 1 to 4 exhibit stronger host-guest interactions than the others, indicating their stronger structure-directing effect toward STW framework. This result agrees with the experimental fact that OSDAs 1 to 4 could direct the formation of STW-type zeolites in experiment while the others in Group A could not. 1, 2, 3, 4, 5-Pentamethylimidazolium (OSDA 1) exhibits the strongest host-guest interaction energy (-154.2 kcal (mol OSDA) -1), and 2-phenyl-1, 3-diethylimidazolium (OSDA 5) exhibits the weakest (-89.6 kcal (mol OSDA) -1) because of the steric hindrance of its phenyl rings.

|
Download:
|
| Fig. 2. Distribution of (A) 1, 2, 3, 4, 5-pentamethylimidazolium cation (OSDA 1 in Group A), (B) 1, 2, 3, 5-tetramethylpyrazolium cation (OSDA 12 in Group B), and (C) (butane-1, 4-diyl)bis(2, 3, 4, 5-tetramethylimidazolium) (OSDA 21 in Group C) within the channels of STW. Si, C, and N atoms are shown as yellow, grey, and blue, respectively. O and H atoms are omitted for clarity. | |
Fig. 3A shows the plot of the average Mülliken charges assigned to nitrogen atoms versus the dipole moments of the OSDA cations in Group A derived from DFT calculations. All successful OSDAs are located at the bottom left of the plot. That means the OSDAs with more negative charges on nitrogen atoms and smaller dipole moments are easier to direct the formation of STW-type zeolites than the others in Group A. This makes sense because the nitrogen atoms with many negative charges could interact strongly with the positively charged zeolite framework, and the small dipole moments imply a relatively even distribution of atomic charges in OSDA cations, which matches the shape of the host stw cage.

|
Download:
|
| Fig. 3. Average Mülliken charges on nitrogen atoms versus dipole moments of OSDAs in (A) Group A, (B) Group B and (C) Group C. Successful, unsuccessful, and predicted OSDAs for the synthesis of STW-type zeolites are shown in green, red and black circles. | |
On the basis of this discovery, we have designed several monoquatenary imidazolium-based cations according to the OSDAs in Group A. None of these cations has been used for the synthesis of STW. By calculating their atomic charge distributions and dipole moments, we find three new imidazolium-based cations in Group A, including 2-ethyl-1, 4, 5-trimethylimidazolium cation (OSDA 22), 1-ethyl-2, 4-dimethylimidazolium cation (OSDA 23), and 2-ethyl-1, 4-dimethylimidazolium cation (OSDA 24), which possess negative charges and dipole moments comparable to successful OSDAs in Group A (Figs. 3A and 4).Detailed results are listed in Table S1 (Supporting information). Further molecular simulation results reveal their host-guest interaction energies toward STW framework as -151.0, -144.4 and -144.8 kcal (mol OSDA) -1, which are all stronger than that of experimentally successful OSDA 4 (Figs. 2 and 4). This implies that these three cations may be promising OSDAs for the synthesis of STW-type zeolites.

|
Download:
|
| Fig. 4. Predicted OSDA cations that may lead to the formation of STW-type zeolites and their host-guest interaction energies toward STW framework (kcal (mol OSDA) -1). | |
Group B consists of six monoquaternary pyrazolium-based OSDA cations (OSDAs 12 to 17, Fig. 1). The strongest host-guest interactions occur when each stw cavity is occupied by one OSDA cation (Fig. 2B). 1, 2, 3, 5-Tetramethylpyrazolium (OSDA 12) exhibits the strongest host-guest interaction with STW, which is the only successful OSDA in Group B that can direct the formation of STW in experiment. 1-Ethyl-2, 3-dimethylpyrazolium (OSDA 14) and 1-ethyl-2, 5-dimethylpyrazolium cations (OSDA 15) are isomers of OSDA 12, but they could not direct the formation of STW. This is due to the differences in not only their shapes but also the atomic charge distribution in these cations. The average Mülliken charge on nitrogen atoms in OSDA 12 is -0.013 e, which is more negative than those on other OSDAs in Group B (Fig. 3B). This agrees well with the OSDAs in Group A, where cations with more negative charges on nitrogen atoms are better OSDAs for STW.
Similarly, based on this discovery, we have designed several monoquatenary pyrazolium-based cation according to OSDA 12, the best OSDA in this group. None of these cations has been used for the synthesis of STW. By calculating their atomic charge distributions and dipole moments, we identify 1, 2, 3, 4, 5-pentamethylpyrazolium (OSDA 25, Fig. 4), which possesses negative charges and dipole moment comparable to experimentally successful OSDA 12 (Fig. 3C). Detail results are listed in Table S2 (Supporting information). Further molecular simulation results reveal its host-guest interaction energy toward STW framework as -137.8 kcal (mol OSDA) -1, which is even stronger than that of OSDA 12 (Figs. 2 and 4). This implies that 1, 2, 3, 4, 5-pentamethylpyrazolium may be another promising OSDA for the synthesis of STW-type zeolites.
Group C consists of four diquaternary imidazolium-based cations that have been used to synthesize STW-type zeolites. In our study, this is the only group whose members are all able to direct the formation of STW in experiment without producing other types of zeolites as side products. The strongest host-guest interactions occur when three OSDA cations are loaded into each unit cell of STW and each OSDA cation rides across two adjacent stw cavities. These cations are stacked in a helical way matching the helical channels of STW and the angles between the planes of the two imidazolium rings in these cations are close to 60, which approximately equals the angles between two adjacent stw cavities (Fig. 2C). From OSDAs 18 to 20, the two imidazolium rings are linked by four, five, and six methylene groups, and the corresponding host-guest interactions decrease with the lengths of the linkers (-318.3, -317.0 and -292.8 kcal (mol OSDA) -1, respectively). This result agrees well with the report by Camblor and co-workers [33]. (Butane-1, 4-diyl)bis(2, 3, 4, 5-tetramethylimi-dazolium) (OSDA 21), which consists of four more methyl groups attached to the imidazolium rings than OSDA 18, exhibits a host-guest interaction stronger than all the others, indicating the importance of the match in size between OSDAs and zeolite channels.
According to these successful OSDAs in Group C, we design some new diquatenary imidazolium-based cations, in which adjacent imidazolium rings are connected by one, two, and three methylene groups, namely methylenebis(2, 3-dimethylimidazo-lium) (OSDA 26), (ethane-1, 2-diyl)bis(2, 3-dimethylimidazolium) (OSDA 27), and (propane-1, 3-diyl)bis(2, 3, 4, 5-tetramethylimidazo-lium) (OSDA 28). All these OSDAs exhibit negative charges on nitrogen atoms and small dipole moments (Fig. 3C). Detailed results are listed in Table S3 in Supporting information. Molecular simulation results show that all these three OSDAs exhibit stronger host-guest interactions than experimentally successful OSDA 19 in this group (Fig. 4). In particular, OSDA 28 possess stronger host-guest interaction toward STW than all experimentally successful OSDAs involved in this study. According to these results, we predict that OSDAs 26 to 28 should be another group of promising structure-directing agents for the synthesis of STW-type zeolites.
Although the experimentally successful OSDA cations studied in this work belong to three different groups, they all exhibit negative charges on nitrogen atoms and small dipole moments. Since calculating the atomic charges and dipole moments of OSDA cations is much easier than calculating the host-guest interactions, our findings provide a new route to the design of unprecedented OSDAs at very low computational costs. By estimating the structure-directing capability of candidate organic cations, we are able to identify the most promising OSDAs toward the rational synthesis of specific zeolite frameworks.
Declaration of competing interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21622102, 21621001 and 21920102005), the National Key Research and Development Program of China (No. 2016YFB0701100), the National 111 Project (No. B17020), Program for JLUSTIRT and High Performance Computing Center of Jilin University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.01.016.
| [1] |
V. Van Speybroeck, K. Hemelsoet, L. Joos, et al., Chem. Soc. Rev. 44 (2015) 7044-7111. DOI:10.1039/C5CS00029G |
| [2] |
B.M. Weckhuysen, J. Yu, Chem. Soc. Rev. 44 (2015) 7022-7024. DOI:10.1039/C5CS90100F |
| [3] |
Y. Li, L. Li, J.H. Yu, Chem 3 (2017) 928-949. DOI:10.1016/j.chempr.2017.10.009 |
| [4] |
S.F. Zhao, X.T. Yao, B.H. Yan, et al., Chin. Chem. Lett. 28 (2017) 1318-1323. DOI:10.1016/j.cclet.2017.03.023 |
| [5] |
M. Dusselier, M.E. Davis, Chem. Rev. 118 (2018) 5265-5329. DOI:10.1021/acs.chemrev.7b00738 |
| [6] |
C. Xu, C. Zhou, S. Wang, A.S. Huang, Chin. Chem. Lett. 30 (2019) 1204-1206. DOI:10.1016/j.cclet.2019.01.016 |
| [7] |
R.F. Lobo, S.I. Zones, M.E. Davis, J. Inclusion Phenom. Mol. Recognit. Chem. 21 (1995) 47-78. |
| [8] |
M. Moliner, F. Rey, A. Corma, Angew. Chem. Int. Ed. 52 (2013) 13880-13889. DOI:10.1002/anie.201304713 |
| [9] |
J. Li, A. Corma, J. Yu, Chem. Soc. Rev. 44 (2015) 7112-7127. DOI:10.1039/C5CS00023H |
| [10] |
Y. Li, H. Cao, J. Yu, ACS Nano 12 (2018) 4096-4104. DOI:10.1021/acsnano.8b02625 |
| [11] |
P. Wagner, Y. Nakagawa, G.S. Lee, et al., J. Am. Chem. Soc. 122 (2000) 263-273. DOI:10.1021/ja990722u |
| [12] |
A. Rojas, O. Arteaga, B. Kahr, M.A. Camblor, J. Am. Chem. Soc. 135 (2013) 11975-11984. DOI:10.1021/ja405088c |
| [13] |
L. Gomez-Hortiguela, F. Cora, C.R. Catlow, J. Perez-Pariente, J. Am. Chem. Soc. 126 (2004) 12097-12102. DOI:10.1021/ja0481023 |
| [14] |
R. Garcia, L. Gomez-Hortiguela, I. Diaz, et al., Chem. Mater. 20 (2008) 1099-1107. DOI:10.1021/cm702098j |
| [15] |
P. Lu, L. Gomez-Hortiguela, M.A. Camblor, Chem. Eur. J. 25 (2019) 1561-1572. DOI:10.1002/chem.201804973 |
| [16] |
G. Sastre, A. Cantin, M.J. Diaz-Cabanas, A. Corma, Chem. Mater. 17 (2005) 545-552. DOI:10.1021/cm049912g |
| [17] |
R.M. Shayib, N.C. George, R. Seshadri, et al., J. Am. Chem. Soc. 133 (2011) 18728-18741. DOI:10.1021/ja205164u |
| [18] |
R. Pophale, F. Daeyaert, M.W. Deem, J. Mater. Chem. A 1 (2013) 6750-6760. DOI:10.1039/c3ta10626h |
| [19] |
D. Jo, S.B. Hong, Angew. Chem. Int. Ed. 58 (2019) 13845-13848. DOI:10.1002/anie.201909336 |
| [20] |
F. Daeyaert, F. Ye, M.W. Deem, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 3413-3418. DOI:10.1073/pnas.1818763116 |
| [21] |
F. Daeyaert, M.W. Deem, J. Mater. Chem. A 7 (2019) 9854-9866. DOI:10.1039/C8TA11913A |
| [22] |
Database of Zeolite Structures, http://www.iza-structure.org/databases/(Accessed 25 August, 2018).
|
| [23] |
L. Tang, L. Shi, C. Bonneau, et al., Nat. Mater. 7 (2008) 381-385. DOI:10.1038/nmat2169 |
| [24] |
A. Rojas, M.A. Camblor, Angew. Chem. Int. Ed. 51 (2012) 3854-3856. DOI:10.1002/anie.201108753 |
| [25] |
L. Shi, T.L. Yu, S. Lin, et al., Chem. J. Chin. Univ. 36 (2015) 1467-1471. |
| [26] |
N. Zhang, L. Shi, T.T. Yu, et al., J. Solid State Chem. 225 (2015) 271-277. DOI:10.1016/j.jssc.2014.12.031 |
| [27] |
S.K. Brand, J.E. Schmidt, M.W. Deem, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 5101-5106. DOI:10.1073/pnas.1704638114 |
| [28] |
D. Jo, S.B. Hong, Chem. Commun. 54 (2018) 487-490. DOI:10.1039/C7CC08818C |
| [29] |
P. Lu, L. Gomez-Hortiguela, L. Xu, M.A. Camblor, J. Mater. Chem. A 6 (2018) 1485-1495. DOI:10.1039/C7TA10002G |
| [30] |
J.E. Schmidt, M.W. Deem, M.E. Davis, Angew. Chem. Int. Ed. 53 (2014) 8372-8374. DOI:10.1002/anie.201404076 |
| [31] |
F. Daeyaert, M.W. Deem, Mol. Phys. 116 (2018) 2836-2855. DOI:10.1080/00268976.2018.1492747 |
| [32] |
Materials Studio, R2, Dassault Systèmes BIOVIA, 2017.
|
| [33] |
P. Dauber-Osguthorpe, V.A. Roberts, D.J. Osguthorpe, et al., Proteins:Struct. Funct. Genet. 4 (1988) 31-47. DOI:10.1002/prot.340040106 |
| [34] |
B. Hammer, L.B. Hansen, J.K. Norskov, Phys. Rev. B 59 (1999) 7413-7421. DOI:10.1103/PhysRevB.59.7413 |
| [35] |
R.S. Mulliken, J. Chem. Phys. 23 (1955) 1833-1846. DOI:10.1063/1.1740588 |
| [36] |
A. De Vita, M.J. Gillan, J.S. Lin, et al., Phys. Rev. B:Condens. Matter. Phys. 46 (1992) 12964-12973. DOI:10.1103/PhysRevB.46.12964 |
 2020, Vol. 31
2020, Vol. 31 

