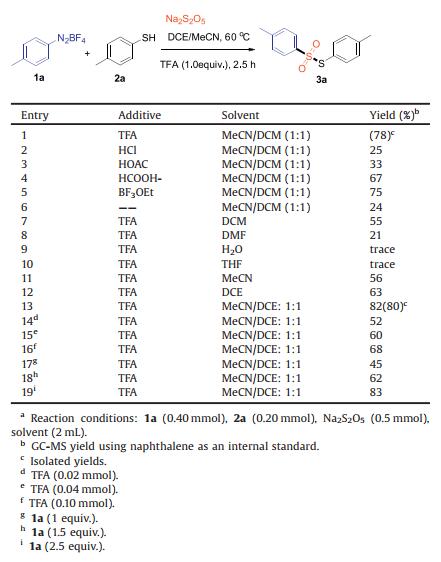
Thiosulfonates have attracted widespread attention because of their potent bactericidal, antimicrobcial and fungicidal activities, together with their various applications in pharmaceuticals, materials, and agriculture [1]. In addition, thiosulfonates have been utilized in many organic transformations as significant synthetic building blocks due to their superior reactivity and stability [2]. In view of the important application value of thiosulfonate, the research on synthesis method of thiosulfonate has also been rapidly developed. In general, direct oxidation of thiols or disulfides [3] and reduction of sulfonyl chlorides [4] afford symmetrical thiosulfonates with the assistance of additional oxidants or reductants. The traditional approaches for the synthesis of unsymmetrical thiosulfonates including reaction of potassium thiosulfonates with diaryliodonium salts [5] and the coupling of sulfides/disulfides with sodium sulfinates [6] (Scheme 1a). In addition, the radical cross-coupling reaction of sulfonyl hydrazides with thiols/disulfides (Scheme 1b) has been developed for the synthesis of unsymmetrical thiosulfonates [7]. However, these methods usually use toxic, unstable sulfenylating agents, some special additives or transition metal catalysts. Recently, Wang and co-workers developed an approach to prepare thiosulfonates with the assistance of BF3-OEt2 (Scheme 1d) [8].Chen and Sun's groups also reported an efficient electrochemical transformation of sulfonyl hydrazides or arylsulfinic acids and thiols to afford thiosulfonates (Scheme 1c) [9]. Despite the great achievements made in this field, the development of a more convenient, sustainable and practical protocol for the generation of unsymmetrical thiosulfonates is still an essential issue.
|
Download:
|
| Scheme 1. A proposed route for unsymmetrical thiosulfonates with the insertion of sulfur dioxide | |
Sulfur dioxide (SO2) as a kind of harmful air pollutant is a major cause of acid rain [10]. However, in laboratory organic synthesis field, sulfur dioxide (SO2) is an important kind of intermediates, showing several advantages including abundance, cheap, and easy conversion to sulfonyl structural motifs viainsertion reactions. Thus, the fixation of sulfur dioxide into organic molecules is meaningful and attractive in synthetic community. In 2010, Willis group developed an elegant aminosulfonylation reaction using DABSO as sulfur dioxide precursor [11]. Subsequently, syntheses of sulfonohydrazides, sulfones and sulfonamides [12] were reported respectively, using similar strategy. In recent reports, Yang and Volla's groups described the synthesis of thiosulfonates using thiols, diazonium salts and DABSO [13]. Sodium metabisulfite is a stable inorganic salt widely exist in nature. It is an ideal sulfur dioxide (SO2) source for the formation of sulfonyl derivatives due to its abundance, and environmentally friendly character. Recently, Na2S2O5 has been developed as a sulfur dioxide surrogate and emerged with significant advantages for assembling sulfonyl derivatives [14]. Inspired by these results, we envisioned that Na2S2O5 might be employed in the construction of unsymmetrical thiosulfonates as well. Herein, we report a novel and efficient strategy for the preparation of unsymmetrical thiosulfonates using sodium metabisulfite as a sulfur dioxide surrogate in the reaction with aryldiazonium salts and thios under transition-metal free conditions.
Initially, the multicomponent reaction of 4-methylphenyldiazonium tetrafluoroborate 1a, 4-methylbenzenethiol 2a, and Na2S2O5 was performed in the presence of TFA (1 equiv.) in CH3CN/DCM (1/1) at 60 ℃ under air (Table 1, entry 1). Pleasingly, the desired product 3a was isolated in 78% yield after 2.5 h (Table 1, entry 1). Furthermore, a series of additive HCl, HOAC, HCOOH, BF3OEt2 were explored (Table 1, entries 1–5). Among the above additives examined, TFA was found to be the most powerful one to give the desired product 3a in 78% yield (Table 1, entry 1). The desired product 3a was only obtained in 24% yield in the absence of the additive (Table 1, entry 6). Next, a range of solvent and mixed solvents were screened (Table 1, entries 7–13), with CH3CN/DCE (1:1) being superior for the synthesis of desired product 3a in 80% yield (Table 1, entry 13). We reduce the amount of TFA, which lead to a decrease in the yields of 3a (Table 1, entry 14–16). We have used 1 equiv., 1.5 equiv. and 2.5 equiv. of aryldiazonium salt 1a, leading to the corresponding products in 45%, 62%, and 83% yields, respectively (Table 1, entry 17–19).
|
|
Table 1 Optimization of the reaction conditions.a |
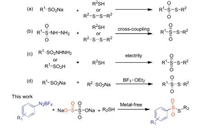
With the optimal conditions in hand, we explore the substrate scope of thiols and the results are summarized in Scheme 2. To our delight, electron-sufficient thiophenols led to good efficiency in the transform action, including methyl (3a) and methoxy (3d) groups. Moreover, the halogen group (F, Br, Cl) substituted thiophenols proceeded smoothly to afford the desired products (3b, 3c, 3e) in good yields. The thiophenols with ortho-, meta-methyl groups on the aromatic rings proceeded well (3f, 3h, 3i), affording the desired products in excellent yields. It was noteworthy that thiophenols bearing an electron withdrawing group (CF3) led to the corresponding product (3j) in 41% yield. Heterocycle thiol such as pyridine-2-thiol afforded the corresponding product (3g) in 33% yield. Next, we transfer our attention to aliphatic thiols. Pleasingly, the more challenging aliphatic thiols were found to be effective and gave the corresponding thiosulfonates in good yields (3k, 3l, 3n, 3o, 3p).

|
Download:
|
| Scheme 2. Substrate scope of the thiols with 2a. Reaction conditions: 1a (0.40 mmol), 2a (0.20 mmol), Na2S2O5 (0.5 mmol), solvent (2 mL). Yields mean isolated yields | |
The bulky tert-butyl thiol could also give the desired product 3m in 25% yield. It is worth mentioning that this method has been successfully applied to the synthesis of cysteine derivatives, and the desired product 3q is obtained in 64% yield.
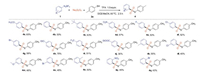
Furthermore, the scope of the various diazonium salts 1 was explored (Scheme 3). Similarly, 2a was successfully reacted with fluoro/chloro/bromo functionalized aryldiazonium tetrafluoroborates and Na2S2O5 to furnish the corresponding products (4e, 4f, 4g, 4l, 4n) with 40%–58% yield. Also, diazonium salts bearing electron-rich group underwent smooth reaction to give desired product (4a, 4c, 4h, 4k, 4m) with moderate yields. 4-Nitroaryldiazonium, 4-trifluoromethyl- and 4-ester-aryldiazonium tetrafluoroborates were compatible with the optimized reaction conditions as well, leading to the corresponding products in 57% (4d), 51% (4i) and 30% yields (4j), respectively. Diazonium salts with ortho substituents groups can also give great products in good yields (4o, 4p, 4q).
|
Download:
|
| Scheme 3. Substrate scope of the diazonium salts with 1a. Reaction conditions: 1 (0.40 mmol), 2a (0.20 mmol), Na2S2O5 (0.5 mmol), solvent (2 mL). Isolated yields | |
To show the scalability of this protocol, diazonium salts 1a reacted with thiols 2a at a 3.0 mmol scale, and the desired thiosulfonate 3a was obtained in 70% yield (0.59 g) (Scheme 4).

|
Download:
|
| Scheme 4. Scale-up reaction | |
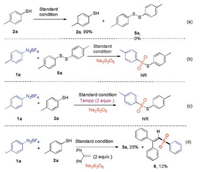
To investigate the possible reaction mechanism, several control experiments were performed (Scheme 5). The reaction of 4-methylbenzenethiol 2a under the standard conditions, disulfide 5a was not observed (Scheme 5a). Disulfide 5a did not react with 4-methylphenyldiazonium tetrafluoroborate 1a and Na2S2O5 under the standard condition (Scheme 5b). This result indicated that the disulfide is not the reaction intermediate for this reaction. When TEMPO (2, 2, 6, 6-tetramethylpiperidine-1-oxyl, 2.0 equiv.) was added to the reaction system, the reaction was completely suppressed (Scheme 5c). This result meant that a radical pathway might be involved. Furthermore, we tried to add ethene-1, 1-diyldibenzene to the reaction, and a 12% yield of the compound 6 was obtained under the optimal conditions (Scheme 5d)
|
Download:
|
| Scheme 5. Control experiment | |
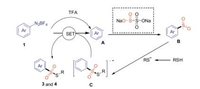
On the basis of the previous reports and above experimental results [15], we have proposed a possible mechanism for this reaction as shown in Scheme 6. First, TFA most likely reacts with diazonium salt to form diazoacetate, and then decomposes to generate the corresponding aryl radical A along, aryl radical A reacts with Na2S2O5 to give the arylsulfonyl radical intermediate B with releasing Na2SO3. Then, the sulfur anion is combined with the arylsulfonyl radical intermediate C to generate the radical anion C. C through a single electron transfer (SET) pathway to afford the desired product thiosulfonates 3 or 4.
|
Download:
|
| Scheme 6. Plausible mechanism | |
In summary, we have developed a convenient and efficient method to construct thiosulfonates under transition-metal free condition through multi-component reaction of aryldiazonium with sodium metabisulphite and thiols. The reaction is easy to handle and tolerates a variety of functional substrates, affording the desired products.
Declaration of competing interestThe authors declare no completing financial interest.
AcknowledgmentsWe gratefully acknowledge the National Natural Science Foundation of China (Nos. 21971174, 21772137, 21672157), the PhD Programs Foundation of PAPD, the Project of Scientific and Technologic Infrastructure of Suzhou (No. SZS201708), the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 16KJA150002), Soochow University, and State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials for financial support.
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at https://doi.org/10.1016/j.cclet.2019.12.032.
| [1] |
(a) J.P. Weidner, S.S. Block, J. Med. Chem. 7(1964) 671-673; (b) S.S. Block, J.P. Weidner, Dev. Ind. Microbiol. 4(1963) 213-217; (c) N.S. Zefirov, N.V. Zyk, E.K. Beloglazkina, A.G. Kutateladze, Sulfur Rep. 14(1993) 223-240; (d) P. Natarajan, Tetrahedron Lett. 56(2015) 4131-4134. |
| [2] |
(a) B.M. Trost, Chem. Rev. 78(1978) 363-382; (b) M.G. Ranasinghe, P.L. Fuchs, Synth. Commun. 18(1988) 227-230; (c) K. Fujiki, E. Yoshida, Synth. Commun. 29(1999) 3289-3294; (d) K. Fujiki, S. Akieda, H. Yasuda, Y. Sasaki, Synthesis 7(2001) 1035-1042; (e) S. Kim, S. Kim, N. Otsuka, I. Ryu, Angew. Chem. Int. Ed. 44(2005) 6183-6186; (f) V. Girijavallabhan, C. Alvarezand, F.G. Njoroge, J. Org. Chem. 76(2011) 6442-6446; (g) Z.H. Peng, X. Zheng, Y.J. Zhang, D.L. An, W.R. Dong, Green Chem. 20(2018) 1760-1764. |
| [3] |
(a) L. Field, T.F. Parsons, J. Org. Chem. 30(1965) 657-659; (b) E. Block, J.O.' Connor, J. Am. Chem. Soc. 96(1974) 3921-3929; (c) Y. Wang, J.H. Espenson, J. Org. Chem. 65(2000) 104-107; (d) V. Nair, A. Augustine, Org. Lett. 5(2003) 543-544; (e) M.T. Cai, G.S. Lv, J.X. Chen, et al., Chem. Lett. 39(2010) 368-369; (f) S. Sobhani, S. Aryanejad, M.F. Maleki, Synlett (2011) 319-322; (g) M. Kirihara, S. Naito, Y. Ishizuka, H. Hanai, T. Noguchi, Tetrahedron Lett. 52(2011) 3086-3089; (h) K. Bahrami, M.M. Khodaei, D. Khaledian, Tetrahedron Lett. 53(2012) 354-358; (i) M. Kirihara, S. Naito, Y. Nishimura, Y. Ishizuka, et al., Tetrahedron 70(2014) 2464-2471; (j) T.X.T. Luu, T.T.T. Nguyen, T.N. Le, J. Spanget-Larsen, F. Duus, J. Sulfur. Chem. 36(2015) 340-350; (k) P.K. Shyam, Y.K. Kim, C. Lee, H.Y. Jang, Adv. Synth. Catal. 358(2016) 56-61. |
| [4] |
(a) Y.L. Yang, B. Rajagopal, C.F. Liang, et al., Tetrahedron 69(2013) 2640-2646; (b) Y. Liu, Y. Zhang, Tetrahedron Lett. 44(2003) 4291-4294; (c) G. Kumaraswamy, R. Raju, V. Narayanarao, RSC Adv. 5(2015) 22718-22723. |
| [5] |
M. Xia, Z.C. Chen, Synth. Commun. 27 (1997) 1309. |
| [6] |
(a) M.D. Bentley, I.B. Douglass, J.A. Lacadie, J. Org. Chem. 37(1972) 333-334; (b) T. Billard, B.R. Langlois, S. Large, et al., J. Org. Chem. 61(1996) 7545-7550; (c) K. Fujiki, N. Tanifuji, Y. Sasaki, T. Yokoyama, Synthesis 3(2002) 343-348; (d) G. Liang, M. Liu, et al., Chin. J. Chem. 30(2012) 1611-1616; (e) G. Liang, J. Chen, J. Chen, et al., Tetrahedron Lett. 53(2012) 6768-6770. |
| [7] |
(a) N. Taniguchi, J. Org. Chem. 80(2015) 1764-1770; (b) G.Y. Zhang, S.S. Lv, A. Shoberu, J.P. Zou, J. Org. Chem. 82(2017) 9801-9807; (c) Z.H. Peng, X. Zheng, Y.J. Zhang, D.L. An, W.R. Dong, Green Chem. 20(2018) 1760-1764; (d) Q. Chen, Y.L. Huang, X.F. Wang, J.W. Wu, G.D. Yu, Org. Biomol. Chem. 16(2018) 1713-1719. |
| [8] |
L. Cao, S.H. Luo, K. Jiang, Org. Lett. 20 (2018) 4754-4758. |
| [9] |
(a) Z.Y. Mo, T.R. Swaroop, Z.F. Chen, et al., Green Chem. 20(2018) 4428-4432; (b) X.F. Zhang, C. Cui, Y.H. Zhang, et al., Adv. Synth. Catal. 361(2019) 2014-2019. |
| [10] |
(a) M. Luo, X.H. Zhang, D. Darensbourg, Acc. Chem. Res. 49(2016) 2209-2219; (b) G. Adaros, H.J. Weigel, H.J. Jauger, New Phytol. 108(1988) 67-74. |
| [11] |
B. Nguyen, E.J. Emmett, M.C. Willis, J. Am. Chem. Soc. 132 (2010) 16372-16373. |
| [12] |
(a) F.S. He, Y.Q. Wu, X.F. Li, H.G. Xia, J. Wu, Org. Chem. Front. 6(2019) 1873-1878; (b) X.F. Wang, M. Yang, W.L. Xie, X.N. Fan, Jie Wu, Chem. Commun. 55(2019) 6010-6013; (c) J. Zhang, W.L. Xie, S.Q. Ye, J. Wu, Org. Chem. Front. 6(2019) 1863-1867; (d) Y. Zong, Y.M. Lang, M. Yang, et al., Org. Lett. 21(2019) 1935-1938; (e) S.Q. Ye, D.Q. Zheng, J. Wu, G.Y.S. Qiu, Chem. Commun. 55(2019) 2214-2217; (f) X.F. Wang, H.Z. Li, G.Y.S. Qiu, J. Wu, Chem. Commun. 55(2019) 2062-2065; (g) D.Q. Zheng, J. Yu, J. Wu, Angew. Chem. Int. Ed. 55(2016) 11925-11929; (h) F. Liu, J.Y. Wang, B. Jiang, et al., Angew. Chem. Int. Ed. 56(2017) 15570-15574; (i) K.D. Zhou, J. Zhang, G.Y.S. Qiu, J. Wu, Org. Lett. 21(2019) 275-278; (j) H.J. Chen, M.L. Liu, G.Y.S. Qiu, J. Wu, Adv. Synth. Catal. 361(2019) 146-150; (k) S. Ye, J. Wu, Chem. Commun. 48(2012) 7753-7755; (l) X.Y. Qin, L. He, J. Li, B. Jiang, et al., Chem. Commun. 55(2019) 3227-3230; (m) S. Liu, K. Chen, W.J. Hao, et al., J. Org. Chem. 84(2019) 1964-1971; (n) Z.J. Shen, Y.N. Wu, C.L. He, et al., Chem. Commun. 54(2018) 445-448; (o) T.H. Zhu, X.C. Zhang, K. Zhao, T.P. Loh, Org. Chem. Front. 6(2019) 94-98; (p) T.H. Zhu, X.C. Zhang, X.L. Cui, et al., Adv. Synth. Catal. 361(2019) 1-7. |
| [13] |
(a) G. Li, Z. Gan, K. Kong, X. Dou, D. Yang, Adv. Synth. Catal. 361(2019) 1808-1814; (b) A.M. Nair, S. Kumar, L. Halder, C.M.R. Volla, Org. Biomol. Chem. 17(2019) 5897-5901. |
| [14] |
(a) X.X. Gong, J.H. Chen, L.F. Lai, et al., Chem. Commun. 54(2018) 11172-11175; (b) H.T. Dang, V.T. Nguyen, V.D. Nguyen, H.D. Armana, O.V. Larionov, Org. Biomol. Chem. 16(2018) 3605-3609; (c) M. Wang, J.Y. Zhao, X.F. Jiang, ChemSusChem 12(2019) 3064-3068; (d) X.X. Gong, M.J. Wang, S.Q. Ye, J. Wu, Org. Lett. 21(2019) 1156-1160; (e) G.Y.S. Qiu, K.D. Zhou, J. Wu, Chem. Commun. 54(2018) 12561-12569; (f) M. Wang, Q.L. Fan, X.F. Jiang, Green Chem. 20(2018) 5469-5473. |
| [15] |
(a) U.M.V. Basavanag, A. Dos Santos, L. El Kaim, R. Gamez Montano, L. Grimaud, Angew. Chem. Int. Ed. 52(2013) 7194-7197; (b) D. Koziakov, A.J.V. Wangelin, Org. Biomol. Chem. 15(2017) 6715-6719; (c) F.P. Crisostomo, T. Martin, R. Carrillo, Angew. Chem. Int. Ed. 53(2014) 2181-2185; (d) M. Hartmann, Y. Li, A. Studer, J. Am. Chem. Soc. 134(2012) 16516-16519; (e) R. Huisgen, L. Krause, Ann. Chem. 574(1951) 157-171; (f) D. Koziakov, G.J. Wu, A.J. von Wangelin, Org. Biomol. Chem. 16(2018) 4942-4953; (g) N. Naveen, S. Sengupta, S. Chandrasekaran, J. Org. Chem. 83(2018) 3562-3569; (h) S.L. Yi, M.C. Li, X.Q. Hu, W.M. Mo, Z.L. Shen, Chin. Chem. Lett. 27(2016) 1505-1508; (i) H.X. Xu, Q.C. Wang, Chin. Chem. Lett. 30(2019) 337-339; (j) K.B. Ouyang, W. Hao, W.X. Zhang, Z.F. Xi, Chem. Rev. 115(2015) 12045-12090; (k) Q.J. Wang, Y.J. Su, L.X. Li, H.M. Huang, Chem. Soc. Rev. 45(2016) 1257-1272; (l) D. Koziakov, A.J. von Wangelin, Org. Biomol. Chem. 15(2017) 6715-6719. |
 2020, Vol. 31
2020, Vol. 31