b Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China;
c School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
d School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
Green and sustainable chemistry has put forward the requirement of replacing traditional non-renewable resources with clean, inexpensive and abundant natural resources such as air [1] and natural plant extracts [2]. In response to this requirement, visible light photocatalytic oxidation using dioxygen has become a hot spot in synthetic chemistry research in recent years [3]. However, noble metals and/or ecologically unfriendly photocatalysts are needed in most photocatalytic oxidation processes, which not only increases production costs, but also generates unnecessary chemical wastes, resulting in complex separation and purification steps and environmental pollution. Compared with pure oxygen, ambient air is a better oxidant in that it is safer, cheaper, and more readily available [4]. Therefore, developing the novel visible-light photocatalytic oxidation which could be conducted with ambient air as the terminal oxidant under photocatalyst-free conditions is highly desirable [5], and great efforts have been made to develop various green processes to avoid the use of hazardous chemicals and to use abundant natural raw materials for organic synthesis. Among them, the chemical process promoted by natural acid has yielded fruitful results [6].
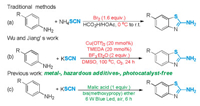
2-Aminobenzothiazoles represent an important class of N-heterocycles [7] whose valuable pharmacological and biological activities make them privileged scaffolds in research and drug developments [8]. A tremendous amount of effort has been paid towards developing novel methods for their preparation [9]. Among the established synthetic approaches, the cascade oxidative coupling/cyclization of aromatic amines with thiocyanate salts is an ideal choice for the construction of such compounds given the cheapness and readily availability of raw materials. However, the traditional method generally requires the usage of excessive toxic molecular bromine (or other halogenated oxidant) and corrosive solvent (such as hydrochloric acid, acetic acid), which not only leads to high manufacturing cost but also causes series environmental problems (Scheme 1a). Recently, Wu and Jiang pioneered the copper-catalyzed oxidative thiocyanation of anilines and KSCN with molecular oxygen as an oxidant and BF3·Et2O as the promoter in dimethyl sulfoxide (DMSO) at 100 ℃ (Scheme 1b) [10]. From a practical and safety point of view, the risk of employing pure O2 and explosive DMSO at high temperature, and the usage of hazardous synthetic acid and metal catalysts largely restrict its application for large-scale industrial manufactures.
|
Download:
|
| Scheme 1. Preparation of 2-aminobenzothiazoles through cascade reaction. | |
Guided by the 12 principles of green and sustainable chemistry [11], herein we report a practical protocol for the preparation of 2-aminobenzothiazoles through visible-light-initiated (Principle 6th, increase energy efficiency) malic acid-promoted (Principle 7th, use of renewable feedstocks) cascade (Principle 8th, reduce derivatives) coupling and cyclization (Principle 2nd, atom economy) of aromatic amines and KSCN by using ambient air as the sole oxidant (Principle 4th, designing safer chemicals) in eco-friendly bis (methoxypropy) ether (Principle 5th, safer solvents) under metal-, hazardous additive-, photocatalyst-free (Principle 1st, prevent waste) conditions (Scheme 1c).
Our preliminary exploration started with 4-toluidine (1a) as a template substrate. Treatment of 1a, NaSCN (2 equiv.), and HOAc (2 equiv.) in bis(methoxypropy)ether (BME, 1 mL) under ambient air by the irradiation of a 6 W blue LED lamp led to the production of the expected 6-methylbenzo[d]thiazol-2-amine (2a) in 25% NMR yield (Table 1, entry 1). Next, the screening of thiocyanate salts (entries 1–3) revealed that the KSCN was the optimal thiocyanation source (entry 2). A series of natural acids (entries 4–8), include glycolic acid, lactic acid, malic acid, oxalic acid and citric acid were subjected to investigation, and malic acid (entry 6) was found to be the best choice for this present reaction. An optimization of the solvents (entries 9–14) illustrated that none of the other solvents was superior to BME. Finally, the impact of varying the concentration of 1a, the amount of KSCN and malic acid were investigated. Decreasing the concentration of 1a resulted in no improvement in the reaction efficiency (entry 15), whereas a slightly lower yield of 2a was observed when the concentration of 1a was increased to 0.4 mmol/mL (entry 16). Altering the amount of KSCN did not improve the yield of 2a (entries 17 and 18). Increasing the loading of malic acid to 3 equiv. did not provide any significant changes in reaction outcome (entry 19). Reducing the amount of malic acid to 1 equiv. was feasible; however, further decreasing to 0.8 equiv. resulted in a lower yield (entries 20 and 21). No conversion of 4-toluidine was observed in the absence of a Brønsted acid (entry 22). No improvement in the photocatalytic reaction was detected when pure oxygen gas was used instead of ambient air (entry 23). When the reaction was conducted under nitrogen atmosphere, no reaction occurred (entry 24). Only a trace amount of 2a was formed while using 6 W green LED lamp as the light source (entry 25). Conducting the reaction under the radiation of sunlight led to much lower reaction efficiency (entry 26). The present transformation did not proceed under the dark condition, indicating that this present reaction involves a visiblelight-induced mechanism (entry 27).
|
|
Table 1 Optimization of reaction conditions.a |
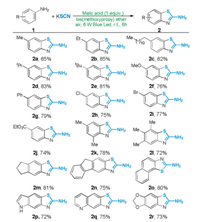
After establishing the optimized conditions (Table 1, entry 20) to access the 2-aminobenzothiazoles, the substrate scope was investigated (Scheme 2). A series of anilines with electrondonating (2a–2f), -neutral (2g), and -withdrawing (2h–2j) groups were well-tolerated and provided the expected products in good yields. Importantly, the substrates containing synthetic valuable functional-groups such as chloro-, bromo-, and ester groups were also well compatible in the current reaction, which were useful for further functionalizations of the 2-aminobenzothiazole moiety. Both di- and tri-substituted anilines are suitable substrates that underwent the transformation smoothly to deliver the desired products (2k and 2l) in good yields. The cascade coupling/ cyclization reactions of polycyclic aromatic amines and KSCN were possible and proceeded with good yields (2m–2o), thus enhancing the substrate scope of the developed reaction. Impressively, our present protocol was further extended to the substrates of heteropolycyclic compounds. The reaction of 5-aminoindole, 6- quinolylamine and 5-aminobenzodioxole afforded the aminothiazole products (2p–2r) in 72%–75% yields.
|
Download:
|
| Scheme 2. Reaction Scope. Conditions: 1 (0.3 mmol), KSCN (0.6 mmol), malic acid (0.3 mmol), BME (1.5 mL), 6 W blue LED lamp, r.t. Isolated yields were reported. | |
The synthetic applicability of the present reaction was further explored (Scheme 3). In the small-scale reaction, a concentration of 0.2 mol/L of the substrate 1 was required, and the increasing of substrate 1 in concentration would decrease the reaction efficiency. Much to our delight, decreasing the amount of BME had virtually no effect on the yield. The cascade reaction of 4- toluidine (10 mmol) with a 5-fold increase in the concentration of 1a provided the expected product 2a in 81% yield (1.29 g). In addition, the pure 2-aminobenzothiazole 2a could be readily collected through extraction.

|
Download:
|
| Scheme 3. Large-scale synthesis of 2a. | |
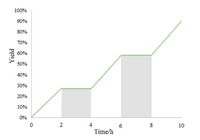
In order to understand the mechanism, several control experiments were performed (Scheme 4). Firstly, the photocatalytic reaction was suppressed in the presence of a radical inhibitor (TEMPO or BHT) (Scheme 4a). The radical capture experiment with 1, 1-diphenylethylene provided the radical adduct 3a (Scheme 4b). These experimental results suggested that this photocatalytic reaction likely proceeded through a free-radical pathway. While conducting the reaction with 2 equiv. of TBHP or H2O2 as an oxidant under nitrogen atmosphere, a trace amount of 2a was observed (Scheme 4c). Under the standard conditions, 9, 10-dimethylanthracene 4a was converted to the endoperoxide product 4b through [4 + 2] cycloaddition reaction (Scheme 4d). When 2 equiv. of singlet oxygen scavenger (1, 4-diazabicyclo[2.2.2]octane, DABCO) was added to the reaction mixture, only a trace amount of 2a was formed (Scheme 4e). Taken together, these results suggested that the singlet oxygen might be a reactive intermediate in the transformation. The lighting time profiles of the cascade reaction showed that this reaction was fully suppressed in the absence of visible-light irradiation (Fig. 1). These results suggested that the continuous visible-light irradiation was necessary for the transformation.

|
Download:
|
| Scheme 4. Control experiments. | |
|
Download:
|
| Fig. 1. On/off control experiments. | |
A plausible reaction mechanism for the light-initiated cascade reaction based on the above experimental results and related literature was illustrated in Scheme 5 [12]. Initially, the singlet oxygen 1O2 originated from molecular oxygen in air under the irradiation of visible-light. Meanwhile, the thiocyanic acid was generated in situ by the reaction of thiocyanate salts with malic acid. Subsequently, the active singlet oxygen abstracted one electron from thiocyanic acid to produce a thiocyanate radical and a hydroperoxyl radical. The thiocyanate radical easily reacted with 1a to generate a free-radical intermediate IM1, which underwent dehydro-aromatization to form the 2-thiocyanatoaniline IM2 along with the generation of hydrogen peroxide (detected by a KI-starch test paper) in the presence of hydroperoxyl radicals. Finally, the nucleophilic amino group of IM2 intramolecular attacked the carbon atom of SCN group to generate the target 2-aminobenzothiazole 3.
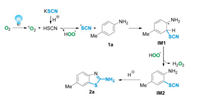
|
Download:
|
| Scheme 5. Plausible mechanism. | |
In summary, we have developed a simple and practical method for the synthesis of 2-aminobenzothiazoles through visible-lightinitiated malic acid-promoted cascade coupling/cyclization of aromatic amines and KSCN with air ambient as an oxidant at room temperature. Mechanistic investigation revealed that the singlet oxygen was produced from molecular oxygen (in air) under the visible-light irradiation, which is pivotal for this cascade reaction to proceed. The protocol is operationally easy and practical with a simple extraction necessary to obtain the target products in good yields and purity for large-scale preparations, thus circumventing the tedious chromatographic purification procedures. Given the readily available materials, the clean conditions (metal-, halogen-, corrosive acid- and photocatalyst-free), high scalability and operational simplicity, this protocol is highly attractive in the field of fine chemistry and pharmaceutical industry.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentWe are grateful for financial support from the Hunan Provincial Natural Science Foundation of China (No. 2019JJ20008).
Appendix A. Supplementary dataSupplementarymaterial relatedtothisarticle canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.011.
| [1] |
(a) W.M. Zhu, W.H. Bao, W.W. Ying, et al., Asian J. Org. Chem. 7 (2018) 337-340; (b) F.L. Zeng, X.L. Chen, S.Q. He, et al., Org. Chem. Front. 6 (2019) 1476-1480; (c) R. Liu, M. Li, W. Xie, et al., J. Org. Chem. 84 (2019) 11763-11773; (d) L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6 (2019) 3950-3955; (e) Z. Gan, Q. Yan, G. Li, et al., Adv. Synth. Catal. 361 (2019) 4558-4567; (f) B. Liu, L. Cheng, P. Hu, et al., Chem. Commun. 55 (2019) 4817-4820; (g) K.J. Liu, J.H. Deng, J. Yang, et al., Green Chem. 22 (2020) 433-438. |
| [2] |
(a) Y. Gu, F. Jerome, Chem. Soc. Rev. 42 (2013) 9550-9570; (b) B. Lai, R. Bai, Y. Gu, ACS Sustain. Chem. Eng. 6 (2018) 17076-17086; (c) G.P. Yang, X. Wu, B. Yu, C. Hu, ACS Sustain. Chem. Eng. 7 (2019) 3727-3732; (d) M. Li, X. Dong, N. Zhang, F. Jérôme, Y. Gu, Green Chem. 21 (2019) 4650-4655; (e) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262. |
| [3] |
(a) D. Xia, Y. Li, T. Miao, P. Li, L. Wang, Green Chem. 19 (2017) 1732-1739; (b) R. Li, X. Chen, S. Wei, et al., Adv. Synth. Catal. 360 (2018) 4807-4813; (c) B.G. Cai, J. Xuan, W.J. Xiao, Sci. Bull. 64 (2019) 337-350; (d) Z. Wang, X. Ji, J. Zhao, H. Huang, Green Chem. 21 (2019) 5512-5516; (e) M. Zhang, M.N. Chen, J.M. Li, N. Liu, Z.H. Zhang, ACS Comb. Sci. 21 (2019) 685-691; (f) J. Wu, Y. Zhang, X. Gong, Y. Meng, C. Zhu, Org. Biomol. Chem.17 (2019) 3507-3513; (g) L.Y. Xie, J.L. Hu, Y.X. Song, et al., ACS Sustain. Chem. Eng. 7 (2019) 19993-19999; (h) Y. Liu, Q.L. Wang, Z. Chen, et al., Chem. Commun. 55 (2019) 12212-12215; (i) Q. Liu, F. Liu, H. Yue, et al., Adv. Synth. Catal. 361 (2019) 5277-5282; (j) G. Li, Q. Yan, Z. Gan, et al., Org. Lett. 21 (2019) 7938-7942; (k) S. He, X. Chen, F. Zeng, et al., Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2019.12.031; (l) W. Ou, R. Zou, M. Han, L. Yu, C. Su, Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2019.12.017; (m) P. Bao, F. Liu, Y. Lv, et al., Org. Chem. Front. 7 (2020) 492-498; (n) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70. |
| [4] |
(a) K.J. Liu, J.H. Deng, T.Y. Zeng, et al., Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.01.036; (b) L.Y. Xie, Y.S. Lu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173; (c) D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40 (2019) 1494-1498; (d) D.Q. Dong, W.J. Chen, D.M. Chen, et al., Chin. J. Org. Chem. 39 (2019) 3190-3198; (e) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725. |
| [5] |
(a) M. Zhang, Q.Y. Fu, G. Gao, et al., ACS Sustain. Chem. Eng. 5 (2017) 6175-6182; (b) L. Zhao, P. Li, X. Xie, L. Wang, Org. Chem. Front. 5 (2018) 1689-1697; (c) L. Zhao, P. Li, H. Zhang, L. Wang, Org. Chem. Front. 6 (2019) 87-93; (d) Y. Han, Y. Jin, M. Jiang, H. Yang, H. Fu, Org. Lett. 21 (2019) 1799-1803; (e) Y.T. Xu, C.Y. Li, X.B. Huang, et al., Green Chem. 21 (2019) 4971-4975. |
| [6] |
(a) J. Yang, J.N. Tan, Y. Gu, Green Chem. 14 (2012) 3304-3317; (b) G. Gao, P. Wang, P. Liu, et al., Chin. J. Org. Chem. 38 (2018) 846-854; (c) M. Sun, J. Jiang, J. Chen, Q. Yang, X. Yu, Tetrahedron 75 (2019) 130456; (d) L. Peng, Z. Hu, Q. Lu, et al., Chin. Chem. Lett. 30 (2019) 2151-2156; (e) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138; (f) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240; (g) X.M. Xu, D.M. Chen, Z.L. Wang, Chin. Chem. Lett. 31 (2020) 49-57. |
| [7] |
(a) L. Wang, D. Xiong, L. Jie, C. Yu, X. Cui, Chin. Chem. Lett. 29 (2018) 907-910; (b) S. Du, C. Pi, T. Wan, Y. Wu, X. Cui, Adv. Synth. Catal. 361 (2019) 1766-1770; (c) K. Sun, X.L. Chen, Y.L. Zhang, et al., Chem. Commun. 55 (2019) 12615-12618; (d) L. Wang, Y. Zhang, M. Zhang, et al., Tetrahedron Lett. 60 (2019) 1845-1848; (e) Q. Huang, L. Zhu, D. Yi, X. Zhao, W. Wei, Chin. Chem. Lett. 31 (2020) 373-376; (f) X. Gong, G. Li, Z. Gan, et al., Asian J. Org. Chem. 8 (2019) 1472-1478; (g) Z. Yang, Z. Song, L. Jie, L. Wang, X. Cui, Chem. Commun. 55 (2019) 6094-6097; (h) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368; (i) J. Sun, Y. Li, Y. Gui, et al., Chin. Chem. Lett. 30 (2019) 569-572; (j) Z. Shen, C. Pi, X. Cui, Y. Wu, Chin. Chem. Lett. 30 (2019) 1374-1378; (k) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30 (2019) 2295-2298; (l) X.X. Meng, Q.Q. Kang, J.Y. Zhang, et al., Green Chem. 22 (2020) 1388-1392; (m) M.Y. Min, R.J. Song, X.H. Ouyang, J.H. Li, Chem. Commun. 55 (2019) 3646-3649; (n) Y.C. Wu, S.S. Jiang, R.J. Song, J.H. Li, Chem. Commun. 55 (2019) 4371-4374; (o) X.P. Ma, C.M. Nong, J. Zhao, et al., Adv. Synth. Catal. 362 (2020) 478-486; (p) H.P. Zhao, G.C. Liang, S.M. Nie, et al., Green Chem. 22 (2020) 404-410; (q) L. Zhu, W. Liao, H. Chang, X. Liu, S. Miao, ChemistrySelect 5 (2020) 829-833; (r) Z. Wang, Q. Liu, X. Ji, G.J. Deng, H. Huang, ACS Catal. 10 (2020) 154-159; (s) Q.Q. Han, G.H. Li, Y.Y. Sun, et al., Tetrahedron Lett. 61 (2020) 151704. |
| [8] |
A. Catalano, A. Carocci, I. Defrenza, et al., Eur. J. Med. Chem. 64 (2013) 357-364. DOI:10.1016/j.ejmech.2013.03.064 |
| [9] |
(a) Y. Xu, B. Li, X. Zhang, X. Fan, J. Org. Chem. 82 (2017) 9637-9646; (b) M. Salah, M. Abdel-Halim, M. Engel, MedChemComm 9 (2018) 1045-1053; (c) T.L. Dadmal, S.D. Katre, M.C. Mandewale, R.M. Kumbhare, New J. Chem. 42 (2018) 776-797. |
| [10] |
H. Jiang, W. Yu, X. Tang, J. Li, W. Wu, J. Org. Chem. 82 (2017) 9312-9320. DOI:10.1021/acs.joc.7b01122 |
| [11] |
(a) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308; (b) S. Peng, D. Hu, J.L. Hu, et al., Adv. Synth. Catal. 361 (2019) 5721-5726; (c) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290; (d) Z. Wang, W.M. He, Chin. J. Org. Chem. 39 (2019) 3594-3595; (e) L. Peng, Z. Hu, Z. Tang, Y. Jiao, X. Xu, Chin. Chem. Lett. 30 (2019) 1481-1487; (f) W.M. He, Y.W. Lin, D.H. Yu, Sci. China Chem. 63 (2020) 291-293; (g) S. Peng, Y.W. Lin, W.M. He, Chin. J. Org. Chem. 40 (2020) 541-542; (h) Y.Y. Peng, Y. Wang, X.Y. Yu, et al., Chem. J. Chin. Univ. 41 (2020) 268-276; (i) Q.W. Gui, X. He, W. Wang, et al., Green Chem. 22 (2020) 118-122. |
| [12] |
(a) X. Zhu, P. Li, Q. Shi, L. Wang, Green Chem. 18 (2016) 6373-6379; (b) Q. Shi, P. Li, X. Zhu, L. Wang, Green Chem. 18 (2016) 4916-4923; (c) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1603; (d) L. Zou, P. Li, B. Wang, L. Wang, Chem. Commun. 55 (2019) 3737-3740; (e) G. Li, Q. Yan, X. Gong, X. Dou, D. Yang, ACS Sustain. Chem. Eng. 7 (2019) 14009-14015. |
 2020, Vol. 31
2020, Vol. 31