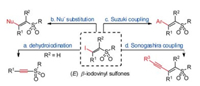
Vinyl sulfones are a type of important organic compounds and have been widely applied in organic synthesis and drug discovery [1, 2]. (E)-β-Iodovinyl sulfones are one of structurally special types of vinyl sulfones (Scheme 1). Their iodine atoms are responsible for many unique transformations, such as: (a) dehydroiodination [3], (b) nucleophilic substitution [4], (c) Suzuki coupling [5], (d) Sonogashira coupling [6]. They have been widely used as versatile synthons for the synthesis of functionalized sulfones.
|
Download:
|
| Scheme 1. (E)-β-Iodovinyl sulfones as versatile synthons. | |
In literature, the iodosulfonylation of alkynes has been recognized as the most practical method for the synthesis of (E)-β-iodovinyl sulfones. The first iodosulfonylation was reported by Truce [3e] in 1971, in which RSO2I (1a) was used as both a sulfonylating reagent and an iodine sourceunder illumination (Scheme 2a). However, this most straightforward procedure lost its appeal soon because RSO2I (1a) are highly instable compounds for preparation and storage. In the past decades, many modified procedures [2] have been developed to use RSO2Na (1b) [3d, 5a–c, 7], RSO2H (1c) [6b, 8], TosMIC (1d) [9], DABCO(SO2)2 (1e) [10] or RSO2NHNH2 (1f) [11] as a sulfonylating reagent in the presence of an iodine source and an oxidant (Scheme 2b). Although each of reagents has its own advantages, they all have their own unavoidable drawbacks. For example, RSO2Na (1b) is the most often used sulfonylating reagent, but this salt usually suffers from the poor solubility in organic solvents. In recent years, RSO2NHNH2 (1f) received much more attention due to its satisfactory stability, solubility and preparation, but its reactions usually suffers from the uses of large amounts of peroxide oxidants under prolonged heating conditions.

|
Download:
|
| Scheme 2. Previous iodosulfonylations and this work. | |
Herein, we report a novel method for the synthesis of (E)-β-iodovinyl arenesulfones (4), in which N, N'-disulfonylhydrazines [(ArSO2NH)2, 3] were introduced as new sulfonylating reagents. As shown in Scheme 2c, the desired product 4 was synthesized efficiently by mixing an alkyne (2), a N, N'-disulfonylhydrazine (3) and NIS (N-iodosuccinimide) together in aqueous THF at room temperature within five minutes.
The mechanism studies [3e, 5a–c, 6b, 7 - 11] indicated that the iodosulfonylations of alkynes similarly went through a radical addition pathway. As shown in Scheme 3, this radical addition was initiated by a sulfonyl radical (RSO2·) [3e] that was generated in situ from a sulfonylating reagent. Therefore, the methodology study for the synthesis of (E)-β-iodovinyl sulfones actually has been became an issue how to conveniently and efficiently generate the sulfonyl radicals.

|
Download:
|
| Scheme 3. Radical addition pathway for the iodosulfonylations of alkynes. | |
Thus, we made a comprehensive investigation for different types of sulfonyl substituted hydrazines. We were surprised to find that, as early as 1970 [12], N, N'-disulfonylhydrazines [Ar1SO2NHNHSO2Ar2 (3)] have been proven to be the most convenient and efficient precursors of the sulfonyl radicals. As shown in Scheme 4, when compound 3 was treated with an oxidant (such as Br2 [12], NBS [12], KMnO4 [12], Chloranil [12], NaOCl [13] or HNO3 [14]), it quickly released N2 via an azo-intermediate (5) to generate two sulfonyl radicals (6). Finally, two sulfonyl radicals were dimerized into a disulfone R1SO2-SO2R2 (7, Ar1 = Ar2 or Ar1≠Ar2). For example, the disulfone Ts-Ts (7a) was produced in 52% yield by treatment of TsNHNHTs (3a) [14b] with HNO3 at room temperature for 1 h. The disulfone PhSO2-SO2Ph (7b) was obtained in 41% yield when (PhSO2NH)2 (3b) was treated with NaOCl at 0 ℃ for 5 min [13]. Remarkably, unsymmetrical disulfone Ts-SO2Ph (7c) was obtained as single product in 67% yield from the oxidation of unsymmetrical TsNHNHSO2Ph (3 h) [14b]. Clearly, these disulfons 7 wereformedby a self-dimerization controlled strongly by the "solvent-cageeffects" [12].

|
Download:
|
| Scheme 4. N, N'-disulfonylhydrazines (3) as precursors of sulfonyl radicals. | |
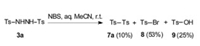
Our investigation also showed that N, N'-disulfonylhydrazines (3) have had several important synthetic applications in recent years [15]. In these works, only the reactions of N-atom in the compounds 3 were emphasized. In fact, N, N'-disulfonyl-hydrazines (3) have not been used as the precursors of sulfonyl radicals in organic synthesis except the simple self-dimerization synthesis of disulfones (7) in nearly half a century. This phenomenon may be caused by the negative influence of the "solvent-cage-effects" on sulfonyl radicals. However, we carefully found that a mixture including the product Ts-Br (8) was obtained when 3a was treated with NBS in aqueous MeCN (Scheme 5) [12]. We strongly believed that the formation of 8 resulted from the fact that a radical Ts·was captured by a radical Br·. This result also indicated that the "solvent-cage-effects" on sulfonyl radicals may be broken by an external radical scavenger.
|
Download:
|
| Scheme 5. Sulfonyl radicals captured by bromine radicals. | |
Thus, we hypothesized that (E)-β-iodovinyl arenesulfones (4) may be synthesized from an alkyne (2, as an external radical scavenger) and a (ArSO2NH)2 (3) in the presence of NIS (as an oxidant and an iodine source). As shown in Table 1, a group of conditional experiments were tested by using PhC≡CH (2a) and TsNHNHTs (3a) as model substrates. When the solution of 2a and 3a in anhydrous MeCN was treated with NIS for 2 h, the desired product 4a was obtained in 9% yield (entry 1). However, the N2 released quickly to give the product 4a in 82% yield within 10 min when the same reaction proceeded in aqueous MeCN (96 vol%) (entry 2). This result indicated that NIS was not strong enough to convert 3a into the corresponding azo-intermediate 5a. The "real oxidant" may be HOI that was formed in situ by hydrolysis of NIS in water. None of the product 4a was obtained when toluene was used as a solvent, possibly due to the poor solubility of the substrates (entry 3). THF was the best solvent to give the product 4a in 92% yield within 5 min (entry 7). The results in entries 8 and 9 indicated that the yield of 4a was decreased by decreasing the amounts of 3a because some of Ts · were converted into Ts-Ts (7a) and Ts-OH (9). Only 7% of 4a was obtained when the reaction proceeded in water because both of substrates were not soluble in water (entry 10). The yield of 4a was improved slightly by addition of 0.5 equiv. of TBAI as a phase-transfer-catalyst (entry 11).
|
|
Table 1 Effects of the solvents, H2O and reaction times.a |
Next, the different oxidants and iodine sources were tested as the alternatives of NIS (Table 2). Although ICl and I2 are the oxidative iodine sources, they showed poor reactivity for this reaction (entries 1 and 2). When the combinations of NaOCl/I2 (entry 3), NaOCl/NaI [16] (entry 4), NCS/I2 (entry 5) or NCS/NaI [17] (entry 6) were used, the N2 released completely within 5 min to give the product 4a in 18%–66% yields. These unsatisfactory results indicated that NaOCl and NCS were good oxidants for the decomposition of TsNHNHTs (3a). However, I2 and NaI were unsuitable iodine sources, possibly because the formation of iodine radicals (I·) from these iodine sources was a slow process. When the mixture of 2a and 3a was treated with the combination of I2/ NIS, 4a was obtained in 90% yield (entry 7).
|
|
Table 2 Effects of the oxidants and iodine sources.a |
At this time, we realized that we may find one of the most efficient methods for the synthesis of (E)-β-iodovinyl arenesulfones (4). To the best of our knowledge, no such method has been reported in literature so far. Thus, the scope of this method was tested under standard conditions (Scheme 6). By fixing TsNHNHTs (3a), different products 4a-4k were obtained in high to excellent yields from aryl ethynes 2a-2k. The product 4l was obtained smoothly in 70% yield from pyridin-2-yl ethyne (2l). Although the product 4m was obtained in 36% yield from cyclohexyl ethyne (2m), only complicated mixtures were obtained from other alkyl ethynes. By fixing phenyl ethyne (2a), all tested disulfonylhydrazines 3a-3g proved to be highly efficient sulfonylating reagents.The desired products 4n-4s were obtained in excellent yields and no comparable electronic effects were observed.

|
Download:
|
| Scheme 6. The scopes of substrates and products. | |
The N, N'-disulfonylhydrazines (3) proved to be so such strong sulfonylating reagents that they reacted smoothly with internal alkynes (2n-2q) to give the tetra-substituted (E)-β-iodovinyl sulfones 4t-4z. Under the influences of the steric and/or electronic effects of the substituents on the internal alkynes, the products 4t and 4u were obtained in relatively low yields. Usually, the hydroxyl group could not survive under the conditions of the existing methods. However, the products 4w-4z bearing the versatile hydroxymethyl groups were synthesized in moderate yields extremely short reaction times and mild conditions were used in our method. Since the products 4w-4z are new compounds, the structure of 4z was determined by X-ray single crystal diffraction and its (E)-configuration was confirmed. Under the standard conditions, the product 4a was prepared on 2-gram scales in 90% yield. However, N, N'-dialkyl sulfonyl-hydrazines have been proved to be unsuitable sulfonylating reagents for our methods.
To further understand the mechanism of this method, more conditional experiments were tested under the standard conditions. As shown in Schemes 7a and b, no differences were observed when the reaction proceeded in the dark or under O2 atmosphere. None of the product 4a was detected in the presence of one equiv of TEMPO (Scheme 7c). These results indicated that our method may go through a radical addition pathway and the tosyl radical (Ts·) is generated by an oxidative decomposition of 3a. As was expected, two products 4a and 4n were obtained when unsymmetrical N, N'-disulfonylhydrazine 3h was used as a substrate (Scheme 7d).

|
Download:
|
| Scheme 7. Further conditional experiments. | |
Under the standard conditions, some of the existing sulfonylating reagents were tested as shown in Schemes 8a–e. The results clearly indicated that all these existing sulfonylating reagents were not suitable for our method.

|
Download:
|
| Scheme 8. The tests for different sulfonylating reagents. | |
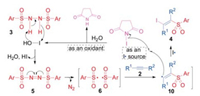
Thus, a possible pathway for our method was proposed as shown in Scheme 9. Initially, NIS was hydrolyzed by H2O to form the stronger oxidant HOI, by which N, N'-disulfonyl-hydrazine (3) was oxidized into the corresponding azo-intermediate (5). The azo-intermediate (5) was then spontaneously decomposed into two sulfonyl radicals (6). When the sulfonyl radical (6) was captured by an alkyne (2), a vinyl radical (10) was formed. Finally, the vinyl radical (10) attacked NIS to get an iodine radical to yield the target product (4). In this pathway, NIS served as the precursors for both oxidant HOI and iodine radical (I·). Since no additional peroxide oxidant needed, our method was performed under extremely convenient and safe conditions.
|
Download:
|
| Scheme 9. A proposed pathway for the formation of 4. | |
In conclusion, a new method for highly efficient synthesis of (E)-β-iodovinyl arenesulfones was developed by simply mixing an alkyne, a N, N'-disulfonylhydrazine and NIS together in aqueous THF for 5 min at room temperature. Its success is mainly due to two distinctive works: (a) N, N'-disulfonyl-hydrazines were introduced as highly efficient sulfonylating reagents; (b) the combinations of N, N'-disulfonylhydrazines/NIS were discovered as highly efficient iodosulfonylating reagent of alkynes.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 21971138 and 21472107).
| [1] |
(a) T.G. Back, K.N. Clary, D. Gao, Chem. Rev. 110 (2010) 4498-4553; (b) M. Nielsen, C.B. Jacobsen, N. Holub, et al., Angew. Chem. Int. Ed. 49 (2010) 2668-2679; (c) Q. Zhu, Y. Lu, Aust. J. Chem. 62 (2009) 951-955; (d) T. Pathak, Tetrahedron 64 (2008) 3605-3628; (e) P.L. Fuchs, T.F. Braish, Chem. Rev. 86 (1986) 903-917. |
| [2] |
(a) Y. Fang, Z. Luo, X. Xu, RSC Adv. 6 (2016) 59661-59676; (b) N.W. Liu, S. Liang, G. Manolikakes, Synthesis 48 (2016) 1939-1973; (c) D.C. Meadows, J. Gervay-Hague, Med. Res. Rev. 26 (2006) 793-814; (d) N.S. Simpkins, Tetrahedron 46 (1990) 6951-6984. |
| [3] |
(a) J. Jia, Y.A. Ho, R.F. Bulow, et al., Chem. -Eur. J. 24 (2018) 14054-14058; (b) M.A. Shameem, K. Esfandiarfard, E. Öberg, et al., Chem. -Eur. J. 22 (2016) 10614-10619; (c) J.R. Bull, N.S. Desmond-Smith, S.J. Heggie, et al., Synlett (1998) 900-902; (d) N. Iwata, T. Morioka, T. Kobayashi, et al., Bull. Inomata, Chem. Soc. Jpn. 65 (1992) 1379-1388; (e) W.E. Truce, G.C. Wolf, J. Org. Chem. 36 (1971) 1727-1732. |
| [4] |
(a) Y. Liang, S.H. Suzol, Z. Wen, et al., Org. Lett. 18 (2016) 1418-1421; (b) T. Zoller, D. Uguen, Eur. J. Org. Chem. (1999) 1545-1550; (c) A. Padwa, D.J. Austin, M. Ishida, et al., J. Org. Chem. 57 (1992) 1161-1169; (d) W.E. Truce, A.W. Borel, P.J. Marek, J. Org. Chem. 41 (1976) 401-402. |
| [5] |
(a) J. Zhang, Z. Liang, J. Wang, et al., ACS Omega 3 (2018) 18002-18015; (b) Y. Sun, A. Abdukader, D. Lu, et al., Green Chem. 19 (2017) 1255-1258; (c) Y. Gao, W. Wu, Y. Huang, et al., Org. Chem. Front. 1 (2014) 361-364. |
| [6] |
(a) X.X. Gu, M.H. Xie, X.Y. Zhao, et al., Chin. J. Chem. 26 (2008) 1625-1629; (b) W. Wei, J. Wen, D. Yang, et al., RSC Adv. 5 (2015) 4416-4419; (c) J.P. Wan, D. Hu, F. Bai, et al., RSC Adv. 6 (2016) 73132-73135. |
| [7] |
(a) N. Taniguchi, Tetrahedron 74 (2018) 1454-1460; (b) R. Kumar, V. Dwivedi, M.S. Reddy, Adv. Synth. Catal. 359 (2017) 2847-2856; (c) N. Taniguchi, Tetrahedron 70 (2014) 1984-1990; (d) T. Sawangphon, P. Katrun, K. Chaisiwamongkhol, et al., Synth. Commun. 43 (2013) 1692-1707; (e) P. Katrun, S. Chiampanichayakul, K. Korworapan, et al., Eur. J. Org. Chem. (2010) 5633-5641; (f) V. Nair, A. Augustine, T.D. Suja, Synthesis (2002) 2259-2265; (g) K.M. Short, C.B. Ziegler Jr., Tetrahedron Lett. 36 (1995) 355-356. |
| [8] |
Y. Tan, S. Jia, F. Hu, et al., J. Am. Chem. Soc. 140 (2018) 16893-16898. DOI:10.1021/jacs.8b09893 |
| [9] |
L. Kadari, R.K. Palakodety, L.P. Yallapragada, Org. Lett. 19 (2017) 2580-2583. DOI:10.1021/acs.orglett.7b00896 |
| [10] |
Y. Xiang, Y. Kuang, J. Wu, Chem. Eur. J. 23 (2017) 6996-6999. DOI:10.1002/chem.201701465 |
| [11] |
(a) Y. Ma, K. Wang, D. Zhang, et al., Adv. Synth. Catal. 361 (2019) 597-602; (b) H. Cui, C. He, D. Yang, et al., Synlett 29 (2018) 830-834; (c) Y. Hou, L. Zhu, H. Hu, et al., New J. Chem. 42 (2018) 8752-8755; (d) C. Tong, B. Gan, Y. Yan, et al., Synth. Commun. 47 (2017) 1927-1933; (e) L. Yang, D. Hu, L. Wei, et al., Phosphorus Sulfur Silicon Relat. Elem. 192 (2017) 1301-1304; (f) G.C. Senadi, B.C. Guo, W.P. Hu, et al., Chem. Commun. (Camb.) 52 (2016) 11410-11413; (g) N.J. Victor, J. Gana, K.M. Muraleedharan, Chem. -Eur. J. 21 (2015) 14742-14747; (h) X. Li, X. Xu, X. Shi, Tetrahedron Lett. 54 (2013) 3071-3074. |
| [12] |
Y. Kohara, M. Kobayashi, H. Minato, Bull. Chem. Soc. Jpn. 43 (1970) 2933-2937. DOI:10.1246/bcsj.43.2933 |
| [13] |
A.T. Maioli, J.P. Anselme, Tetrahedron Lett. 6 (1995) 1221. |
| [14] |
(a) D. Guo, J. Liu, L. Wang, Proc. SPIE 7972, Advances in Resist Materials and Processing Technology XXVIII 79722D, San Jose, California, USA, 2011; (b) E.A. Bartmann, Synthesis (1993) 490-496. |
| [15] |
(a) H.H.C. Lakmal, J.X. Xu, X. Xu, et al., J. Org. Chem. 83 (2018) 9497-9503; (b) X. Wu, B. Liu, Y. Zhang, et al., Angew. Chem. Int. Ed 55 (2016) 12280-12284; (c) U. Ragnarsson, L. Grehn, Tetrahedron Lett. 53 (2012) 1045-1047; (d) M. Fernández, E. Reyes, J.L. Vicario, et al., Adv. Synth. Catal. 354 (2012) 371-376; (e) T. Toma, J. Shimokawa, T. Fukuyama, Org. Lett. 9 (2007) 3195-3197. |
| [16] |
C.R. LeBlond, K. Rossen, F.P. Gortsema, TetrahedronLett. 42 (2001) 8603-8606. DOI:10.1016/S0040-4039(01)01863-9 |
| [17] |
(a) E. Dubost, V. Babin, F. Benoist, et al., Org. Lett. 20 (2018) 6302-6305; (b) Y.D. Vankar, G. Kumaravel, Tetrahedron Lett. 25 (1984) 233-236. |
 2020, Vol. 31
2020, Vol. 31 



