b Research Institute of Petroleum Engineering, Sinopec, Beijing 100101, China
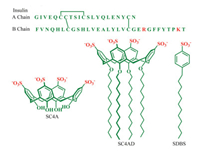
Insulin is a 51-residue protein hormone, composed of A (21-residue) and B (30-residue) chains linked by two disulfide bonds (Fig. 1). It plays a crucial role in regulating glucose metabolism and treating diabetes [1-3]. However, insulin tends to form insoluble amyloid fibrils under certain conditions, such as high concentration, elevated temperature, low pH and high ionic strength, which reduce the insulin potency [4, 5]. For example, amyloid deposits are commonly formed at the site of repeated insulin injection in diabetic patients, thereby causing severe subcutaneous insulin resistance and poor glycemic control [6, 7]. Moreover, the instability of insulin also makes the production, storage and delivery rather difficult [3, 8]. Consequently, it is highly on demand to inhibit insulin fibrillation.
|
Download:
|
| Fig. 1. Amino acid sequence of insulin and molecular structures of SC4A, SC4CE and SDBS. | |
In recent years, many efforts have been devoted to develop inhibiting agents of insulin fibrillation, including natural small molecules [9-11], synthetic compounds [12, 13], peptides [14, 15], ionic liquids [16] and nanoparticles [17, 18]. One core issue of inhibiting insulin (and other proteins) fibrillation is the molecular recognition. Such a challenging problem attracts the research interest of supramolecular chemists too. A series of artificial receptors, such as cyclodextrin [19, 20], calixarene [21], cucurbituril [22, 23] and molecular tweezer [24], have been engaged in inhibiting insulin fibrillation, benefiting from their binding capabilities towards amino acids.
More recently, we proposed a strategy of heteromultivalent recognition by co-assembling of cyclodextrin and calixarene amphiphiles, which not only inhibited the fibrillation of amyloid-β peptides, but also dissolved amyloid-β fibrils [25]. This heteromultivalency strategy improves the binding affinity and selectivity drastically, especially to proteins with multiple and diverse binding sites. We envisaged that the self-assembled heteromultivalency strategy could be easily amenable to other ensembles and targets. Therefore, as a natural evolution of our ongoing program on inhibiting amyloid fibrillation via host-guest interactions, we report here the inhibition of insulin fibrillation by the amphiphilic assembly of p-sulfonatocalix[4]arene tetra dodecyl ether (SC4CE). In comparation, the efficiency of p-sulfonatocalix[4]arene (SC4A, the non-amphiphilic parent compound of SC4CE) and sodium dodecyl benzenesulphonate (SDBS, the mimic building subunit of SC4CE) on inhibiting insulin fibrillation were also investigated. The SC4CE micelle [26] exhibits much better efficiency on inhibiting insulin fibrillation.
The insulin aggregation can be studied by many techniques, such as fluorescence spectroscopy, circular dichroism (CD), turbidity and transmission electron microscopy (TEM) [27]. Insulin fibrillation has been shown to form insoluble aggregates with β-sheet structures [3]. The presence of insoluble protein aggregates leads to an apparent increase in UV–vis absorbance at all wavelengths due to scattering effects [28]. Therefore, the absorbance assay has been extensively employed to monitor the kinetics of insulin aggregation [22, 29, 30]. Herein, the absorbance at 540 nm was measured to monitor the kinetics of insulin aggregation, where native insulin and all of potential inhibitors (SC4CE, SC4A and SDBS) have negligible absorbance (Fig. S1 in Supporting information). The aggregation of 1.0 mg/mL (172.0 μmol/L) insulin was conducted in physiologic salt concentrations using phosphate buffer saline (PBS, 10 mmol/L, pH 7.4). Previous studies have shown that the B-chain residues contribute mainly to insulin aggregation [15, 20]. Lysine (K) and arginine (R) are two B-chain residues of insulin, which can be encapsulated by SC4A with moderate binding affinities (Ka~103 L/mol) [31, 32]. Firstly, 1.0 equiv. SC4CE (172.0 μmol/L, much higher than its critical micelle concentration of 3.5 μmol/L, Fig. S2 in Supporting information) was employed to investigate the inhibition of insulin fibrillation. The fibrillation of insulin follows a nucleation dependent mechanism involving nucleation, elongation, and equilibration phase (Fig. S4 in Supporting information) [3]. As shown in Fig. 2a, the initial nucleation occurs in the first 25 h, and the nucleation phase is followed by the elongation phase. TEM images (Fig. 2d) show that insulin forms large amounts of mature fibrils after the incubation for 100 h. In contrary to the solution of insulin, the absorbance of insulin solution incubated with SC4CE shows no increase during this 135-h kinetic study (Fig. 2a), and no trace of amyloid fibril is found in TEM images (Fig. 2d), demonstrating that the SC4CE micelle completely inhibits the formation of insulin fibrils. In order to examine the advantage of assembly over monomer, SC4A was studied as a control inhibitor. The absorbance of insulin solution incubated with SC4A is slightly lower than insulin (Fig. 2a), indicating that SC4A produces a weak inhibition effect on insulin aggregation under the present condition. SDBS was used as the other control inhibitor to examine the advantage of calixarene scaffold over building subunit. The absorbance of insulin solution incubated with SDBS is lower than insulin after 60 h, suggesting that SDBS partially inhibits insulin aggregation. Intuitively, TEM images (Fig. 2d) show that insulin aggregates into mature fibrils in the presence of SC4A or SDBS, indicating that both SC4A and SDBS are inactive to inhibit insulin fibrillation. CD spectra were then performed to analyze the effect of SC4CE on the secondary structure transition of insulin during the amyloid formation period. The native insulin shows significant α-helical structure with two negative bands at 208 nm and 222 nm [33]. As the fibrillation proceeds, the ellipticities at these two wavelengths increase progressively, indicating the decreased content of α-helix (Fig. 2c) [34]. As expected, the CD spectra of insulin in the presence of SC4CE remain nearly unchanged over a period of 120 h (Fig. 2c), indicating SC4CE inhibits the structural transformation of insulin. The efficient inhibition of SC4CE benefits from two factors. The calixarene cavity donates the recognition site to insulin, more importantly, the amphiphilic assembly provides multivalent recognition which enhances the interaction between the micelle and insulin [35].

|
Download:
|
| Fig. 2. Kinetics of insulin (172.0 μmol/L) aggregation in PBS (10 mmol/L, pH 7.4), (a) monitored by absorbance assay in the absence and presence of SDBS (688.0 μmol/L), SC4A (172.0 μmol/L), SC4CE (172.0 μmol/L), and (b) monitored by absorbance assay with SC4CE of different concentrations. Averages and standard deviation over three replicates are shown. (c) CD spectra of insulin (8.6 μmol/L) in PBS (10 mmol/L, pH 7.4) in the absence and presence of SC4CE (8.6 μmol/L) at different time points. (d) TEM images of insulin in the absence and presence of SDBS, SC4A and SC4CE, incubated in PBS (10 mmol/L, pH 7.4) for 100 h. Scale bars: 200 nm. | |
The kinetics of insulin aggregation in the presence of SC4CE with different concentrations was further investigated. The absorbance assay (Fig. 2b) shows that the SC4CE micelle inhibits the insulin fibril formation at unexpectedly low concentrations. SC4CE (17.2 μmol/L) , which is an order of magnitude lower than the concentration of insulin (172.0 μmol/L), performs the efficacy to prolong the lag time. SC4CE (86.0 μmol/L) is able to completely inhibit the aggregation during the 135-h kinetic study. It is worth noting that although an amount of inhibitors have been reported to inhibit insulin fibrillation, most of them need a large excess [10 , 30] . But for the SC4CE micelle, only 0.5 equiv. is sufficient to inhibit the insulin fibrillation. The SC4CE micelle probably hampers the nucleus formation and thus inhibits further aggregation of insulin [36] . Moreover, when the concentration of SC4CE (1.7 μmol/L) is lower than its critical micelle concentration, SC4CE has almost no inhibition effect on insulin fibrillation.
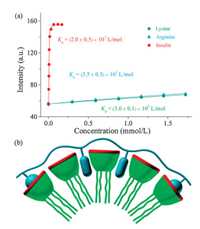
To better understand the inhibition of insulin fibrillation by the SC4CE assembly, we evaluated the interaction between SC4CE and insulin via competitive fluorescence titration [37-39]. Initially, we intended to measure the binding affinity of SC4CE and insulin using lucigenin as a probe [40]. The binding affinity of SC4CE and lucigenin was firstly measured via direct fluorescence titration (Ka = 8.1×105 L/mol, Fig. S7 in Supporting information). By competitively titrating of the complex of SC4CE (10.0 μmol/L) and lucigenin (10.0 μmol/L) with insulin, the fluorescence intensity increases quickly until it reaches equilibrium (Fig. 3a). In contrary to insulin, the fluorescence intensity increases slightly by competitively titrating with lysine or arginine. The associated titration curves are fit according to a n:1 (insulin) and 1:1 (lysine and arginine) binding model to obtain binding affinities. The obtained results suggest that the interaction of SC4CE with insulin (Ka = 2.0×107 L/mol) is much stronger than lysine (Ka = 3.0×102 L/mol) and arginine (Ka = 3.5×102 L/mol). We suspected that other amino acids of insulin besides lysine and arginine also contribute to the strong binding between the SC4CE assembly and insulin. Garcia-Rio et al. have demonstrated that both the host cavity and micellar pseudophase would become the potential recognition sites of guest molecules in amphiphilic SC4A micellar system [41]. In the SC4CE assembly, the micellar pseudophase serves as the other recognition site besides the host cavity. The SC4CE assembly can not only include lysine and arginine into the host cavity, but also complex hydrophobic amino acids (phenylalanine, leucine, valine, etc.) into the micellar pseudophase, realizing finally the heteromultivalent recognition to insulin as illustrated in Fig. 3b.
|
Download:
|
| Fig. 3. (a) Competitive titration curves at λem = 510 nm of lysine, arginine and insulin in the presence of SC4CE (10.0 μmol/L) and lucigenin (10.0 μmol/L) in PB(10 mmol/L, pH 7.4), fit according to a n:1 (insulin) and 1:1 (lysine and arginine)competitive binding model, n = 0.95, λex = 420 nm. Data are from three independent experiments and are presented as mean ± S.E.M. (b) Simplified scheme of heteromultivalent recognition between SC4CE and insulin. | |
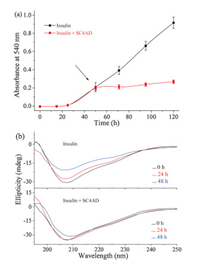
We further examined the disaggregation effect of the SC4CE micelle on insulin fibrils. Insulin was pre-incubated in PBS as described above, and the SC4CE micelle was added after the incubation period. As shown in Fig. 4a, the absorbance of insulin (after the incubation for 50 h) with application of the SC4CE micelle increases slightly rather than declines, which indicates that the SC4CE micelle has no disaggregation effect on preformed insulin fibrils. Nevertheless, the absorbance of insulin with application of the SC4CE micelle is lower than insulin alone, reflecting the inhibition effect of the SC4CE micelle. CD spectra (Fig. 4b) show that the α-helical content of insulin (after the incubation for 100 h) with application of the SC4CE micelle decreases slightly rather than increases, indicating that SC4CE does not have the ability to disaggregate insulin fibrils, which is consistent with the absorbance result. Meanwhile, the decreased content of α-helix with application of the SC4CE micelle is less than insulin alone (Fig. 4b), further demonstrating the inhibition effect of the SC4CE micelle. TEM images (Fig. S8 in Supporting information) also show that mature fibrils can still be observed after incubation with the SC4CE micelle for 50 h. These results suggest that the SC4CE micelle exhibits the inhibition capability of insulin fibrillation even in the presence of "fibril seeds", however, it cannot dissolve mature fibrils. We speculate that the inhibition of insulin fibrillation by SC4CE is a kinetic delay process. That is, SC4CE is capable of inhibiting insulin fibrillation kinetically, but is incapable of disaggregating mature fibrils thermodynamically.
|
Download:
|
| Fig. 4. (a) Kinetics of insulin aggregation in PBS (10 mmol/L, pH 7.4) with or without application of SC4CE, SC4CE was added during the elongation phase (after the incubation for 50 h). Averages and standard deviation over three replicates are shown. (b) CD spectra of insulin in PBS (10 mmol/L, pH 7.4) with or without application of SC4CE at different time points, SC4CE was added during the elongation phase (after the incubation for 100 h). | |
In summary, the SC4CE micelle exhibits much better efficiency on inhibiting insulin fibrillation than SC4A and SDBS, probably derives from the heteromultivalent recognition. The SC4CE micelle can even inhibit insulin aggregation and prolong the nucleation time at low concentration. Mature fibrils cannot be disaggregated in the presence of the SC4CE micelle, indicating that the SC4CE micelle inhibits insulin fibrillation kinetically, while cannot disaggregate insulin fibrils thermodynamically. With such remarkable inhibition effect on insulin fibrillation, the SC4CE assembly has the potential to improve the stability and activity of insulin.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51873090 and 21672112), and the Fundamental Research Funds for the Central Universities, which are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.01.042 .
| [1] |
L. Pirola, A.M. Johnston, E. Van Obberghen, Diabetologia 47 (2004) 170-184. DOI:10.1007/s00125-003-1313-3 |
| [2] |
T.K. Mandal, Am. J. Health. Syst. Pharm. 62 (2005) 1359-1364. DOI:10.2146/ajhp040249 |
| [3] |
M. Groenning, S. Frokjaer, B. Vestergaard, Curr. Protein Peptide Sci. 10 (2009) 509-528. DOI:10.2174/138920309789352038 |
| [4] |
L. Nielsen, R. Khurana, A. Coats, et al., Biochemistry 40 (2001) 6036-6046. DOI:10.1021/bi002555c |
| [5] |
J. Brange, L. Andersen, E.D. Laursen, et al., J. Pharm. Sci. 86 (1997) 517-525. DOI:10.1021/js960297s |
| [6] |
M.R. Nilsson, Amyloid 23 (2016) 139-147. DOI:10.1080/13506129.2016.1179183 |
| [7] |
T. Nagase, K. Iwaya, Y. Iwaki, et al., Am. J. Med. 127 (2014) 450-454. DOI:10.1016/j.amjmed.2013.10.029 |
| [8] |
M. Muzaffar, A. Ahmad, PLoS One 6 (2011) e27906. |
| [9] |
Q..Lazo N.D. Zheng, N.D. Lazo, J. Phys. Chem. B 122 (2018) 2323-2331. DOI:10.1021/acs.jpcb.8b00689 |
| [10] |
S. Choudhary, N. Kishore, R.V. Hosur, Sci. Rep. (2015) 17599. |
| [11] |
A. Arora, C. Ha, C.B. Park, FEBS Lett. 564 (2004) 121-125. DOI:10.1016/S0014-5793(04)00326-6 |
| [12] |
M. Levy-Sakin, M. Shreberk, Y. Daniel, et al., Islets 1 (2009) 210-215. DOI:10.4161/isl.1.3.9609 |
| [13] |
Y. Hong, L. Meng, S. Chen, et al., J. Am. Chem. Soc. 134 (2012) 1680-1689. DOI:10.1021/ja208720a |
| [14] |
N.K. Mishra, K.B. Joshi, S. Verma, Mol. Pharm. 10 (2013) 3903-3912. DOI:10.1021/mp400364w |
| [15] |
M.I. Ivanova, S.A. Sievers, M.R. Sawaya, et al., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 18990-18995. DOI:10.1073/pnas.0910080106 |
| [16] |
T. Takekiyo, E. Yamaguchi, H. Abe, et al., ACS Sustain. Chem. Eng. 4 (2015) 422-428. |
| [17] |
S.H. Li, L.Y. Wang, C.C. Chusuei, et al., Chem. Mater. 27 (2015) 1764-1771. DOI:10.1021/cm504572b |
| [18] |
K..Anand B.G..Badhwar R.. Dubey, B.G. Anand, R. Badhwar, et al., Amino Acids 47 (2015) 2551-2560. DOI:10.1007/s00726-015-2046-6 |
| [19] |
M.N. Shinde, R. Khurana, N. Barooah, et al., J. Phys. Chem. C 121 (2017) 20057-200685. DOI:10.1021/acs.jpcc.7b07286 |
| [20] |
K. Kitagawa, Y. Misumi, M. Ueda, et al., Amyloid 22 (2015) 181-186. DOI:10.3109/13506129.2015.1064818 |
| [21] |
M.N. Shinde, N. Barooah, A.C. Bhasikuttan, et al., Chem. Commun. 52 (2016) 2992-2995. DOI:10.1039/C5CC10159J |
| [22] |
M.J. Webber, E.A. Appel, B. Vinciguerra, et al., Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 14189-14194. DOI:10.1073/pnas.1616639113 |
| [23] |
H.H. Lee, T.S. Choi, S.J. Lee, et al., Angew. Chem. Int. Ed. 53 (2014) 7461-7465. DOI:10.1002/anie.201402496 |
| [24] |
S. Sinha, D.H. Lopes, Z. Du, et al., J. Am. Chem. Soc. 133 (2011) 16958-16969. DOI:10.1021/ja206279b |
| [25] |
Z. Xu, S. Jia, W. Wang, et al., Nat. Chem. 11 (2019) 86-93. DOI:10.1038/s41557-018-0164-y |
| [26] |
K.P. Wang, Y. Chen, Y. Liu, Chem. Commun. 51 (2015) 1647-1649. DOI:10.1039/C4CC08721F |
| [27] |
H.C. Mahler, W. Friess, U. Grauschopf, et al., J. Pharm. Sci. 98 (2009) 2909-2034. DOI:10.1002/jps.21566 |
| [28] |
H.C. Mahler, R. Muller, W. Friess, Eur.J.Pharm.Biopharm. 59 (2005) 407-417. DOI:10.1016/j.ejpb.2004.12.004 |
| [29] |
S.H. Wang, X.Y. Dong, Y. Sun, Biochem. Eng. J. 63 (2012) 38-49. DOI:10.1016/j.bej.2012.02.002 |
| [30] |
J. Jayamani, G. Shanmugam, Eur. J. Med. Chem. 85 (2014) 352-358. DOI:10.1016/j.ejmech.2014.07.111 |
| [31] |
R.E. McGovern, H. Fernandes, A.R. Khan, et al., Nat. Chem. 4 (2012) 527-533. DOI:10.1038/nchem.1342 |
| [32] |
E. Da Silva, A.W. Coleman, Tetrahedron 59 (2003) 7357-7364. DOI:10.1016/S0040-4020(03)01137-2 |
| [33] |
M. Bouchard, J. Zurdo, E.J. Nettleton, et al., Protein Sci. 9 (2000) 1960-1967. DOI:10.1110/ps.9.10.1960 |
| [34] |
L. Nielsen, S. Frokjaer, J. Brange, et al., Biochemistry 40 (2001) 8397-8409. DOI:10.1021/bi0105983 |
| [35] |
Y.C. Pan, H.W. Tian, S. Peng, et al., Chin. Chem. Lett. 28 (2017) 787-792. DOI:10.1016/j.cclet.2016.12.027 |
| [36] |
D.H. Lopes, A. Attar, G. Nair, et al., ACS Chem. Biol. 10 (2015) 1555-1569. DOI:10.1021/acschembio.5b00146 |
| [37] |
L. You, D. Zha, E.V. Anslyn, Chem. Rev. 115 (2015) 7840-7892. DOI:10.1021/cr5005524 |
| [38] |
P. Verwilst, H.R. Kim, J. Seo, et al., J. Am. Chem. Soc. 139 (2017) 13393-13403. DOI:10.1021/jacs.7b05878 |
| [39] |
J. Shin, P. Verwilst, H. Choi, et al., Angew. Chem. Int. Ed. 58 (2019) 5648-5652. DOI:10.1002/anie.201900549 |
| [40] |
D.S. Guo, V.D. Uzunova, X. Su, et al., Chem. Sci. 2 (2011) 1722-1734. DOI:10.1039/c1sc00231g |
| [41] |
S. Fernandez-Abad, M. Pessego, N. Basilio, et al., Chem. -Eur. J. 22 (2016) 6466-6470. DOI:10.1002/chem.201504649 |
 2020, Vol. 31
2020, Vol. 31 

