b CNRS, LCC(Laboratoire de Chimie de Coordination), BP 44099, 31077, Toulouse Cedex 4, France;
c INP-ENSIACET, 4 allée Emile Monso, 31030, Toulouse Cedex, France;
d Université de Toulouse, UPS, ICT-FR 2599, 31062, Toulouse Cedex 9, France
Plants constitute a sustainable source of complex chemical molecules exploited in perfume, food, cosmetic and pharmaceutical industry [1-4]. Among renewable resources, valuable products occurring in relatively high concentration in biomass include fatty acids, their methyl esters or triglycerides (vegetable oils), and terpenes. As relatively small stereo-chemically defined molecules, terpenes are widely used as key building blocks for the industrial hemi-synthetic production of fragrances, flavors or pharmaceuticals [5, 6].
The essential oil of Atlas cedar (Cedrus atlantica) is a unique raw material for the perfume industry [7]. The main component of the essence (75%) is a mixture of three isomeric bicyclic sesquiterpenes, the α-, β- and γ-cis-himachalenes 1a-c, the reactivity of which, and derivatives thereof, having been extensively studied [8-14]. Previous studies have thus shown that himachalene mixtures can be converted to ar-himachalene 2 in the presence of various reagents [15-22]. In this context, we have been interested in synthesizing other aromatic sesquiterpenes such as cadalene 3 and iso-cadalene 4, occurring as biomarkers in various sediments [23-25], and particularly abundant in fossil resins, plant resins and essential oils produced by terrestrial plants [26].
Very few efforts have been dedicated to the total or hemisynthesis of these sesquiterpenes, more particularly iso-cadalene, the total synthesis or chemical modification of which had not been reported. Long after Ruzicka and Seidel's report on the synthesis of cadalene from carvone [27], other routes were reported to occur in low yields (up to 27%) [28-30], the shortest one consisting in a onestep dehydrogenation of cadinene [31]. Nevertheless, the value of cadalene derivatives is readily illustrated by their biological activities, such as the inhibitory effect of 7-hydroxy-3-methoxy-cadalene on 4-(methylinitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice [32], anti-inflammatory and analgesic properties of cadalen-15-oic acid [33], or anticancer cytotoxicity of 7-hydroxy-3-methoxy-cadalene [34].
The hereafter presented results pertain to the development of an unprecedented strategy for the synthesis of cadalene and iso-cadalene, i.e., from the abundant and inexpensive natural feedstock of the α-, β-, γ-himachalene mixture from the essential oil of Atlas cedar.
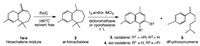
The α-, β-, γ-himachalene mixture was first converted to ar-himachalene 2 by various dehydrogenation agents (selenium, Ni, Pd/C, chloranil (tetrachloro-1, 4-benzoquinone) [35-40]. A solvent-free procedure, using catalytic amount of Pd/C (0.025 wt%) at 160 ℃ for 12 h, was found to afford ar-himachalene 2 with a quantitative yield (Scheme 1). The process was further developed and applied on a multi-gram scale (up to 1 kg), demonstrating the industrial feasibility of the transformation.
|
Download:
|
| Scheme 1. Two-step synthesis of cadalene and iso-cadalene from a himachalene mixture. | |
In an attempt at Friedel-Crafts acylation of 2 with acetyl chloride (AcCl) in the presence of aluminum chloride in dichloromethane (DCM) at room temperature, formation of small amounts of cadalene 3 was observed. In the absence of AcCl under the same conditions, the reaction unexpectedly led to cadalene 3 and dihydrocurcumene 5 with 89% conversion and a selectivity of 21% and 20%, respectively (Table 1, entry 1). Gas chromatography (GC) analysis indicated the formation of ca. 48% of unidentified by-products.
|
|
Table 1 Variation of the reaction conditions (Scheme 1).a |
Further tests were performed by varying the reagent ratios and nature of the solvent (Table 1).
In all cases, the substrate underwent a conversion ranging from 10% to 100%, with a chemoselectivity depending strongly on the reaction parameters. With respect to the above disclosed first results (entries 1 and 2), variation of the reagent stoichiometry (I2 and AlCl3) caused a slight decrease in the selectivity in cadalene 3 while keeping high conversions (entries 3–5). Notably, using a two-fold excess of AlCl3 (vs. I2), formation of minor amounts (6%) of iso-cadalene 4 was observed (entry 5). By contrast, in the absence of AlCl3, only 3 and 5 were produced, with 70% selectivity in the latter product resulting from the opening of the seven-membered ring of ar-himachalene 2 (entry 6).
The effect of solvent was then considered. Changing DCM by dichloroethane (DCE) just gave lower yields in cadalene 3. However, changing DCM by cyclohexane with two molar equivalents of AlCl3 and I2 was found to promote the formation of 72% of by-products, with 14% selectivity in iso-cadalene 4 (entry 7). In the absence of AlCl3, with 2 equiv. of I2, the conversion dropped to 10%, with a mere 2% selectivity in dihydrocurcumene 5 (entry 8).
In the presence of 2 equiv. of AlCl3 only, a 91% conversion and reversed selectivity in iso-cadalene 4 (66%) were obtained, along with 25% of 5 (entry 9). Changing cyclohexane by hexane resulted in both lower conversion and lower yield in 4. With 1 equiv. of AlCl3 (entry 10), a lower conversion of 60% was obtained, albeit with a globally higher 70% selectivity in dihydrocurcumene 5, a valuable natural product also extracted from various sources [42-46]
In the presence of I2, the use of other Lewis acids (FeCl3, ZnCl2, Et2O·BF3, Bi(OTf)3) did not induce any conversion of 2. The same absence of conversion was observed using any Lewis acid in other solvents such as THF, MeCN or EtOH.
The weakly polar products 3, 4 and 5 were purified by column chromatography on silica gel using hexane as eluent (to a ca. 85% purity level only for 4). NMR data of 3 and 4 are similar, except for the secondary isopropyl CH proton resonating at higher field in iso-cadalene 4 (3.06 ppm) than in cadalene 3 (3.80 ppm, due to the deshielding by the closer fused benzenic ring).
Although no quantitative kinetic measurement was carried out, qualitative TLC monitoring showed that under working conditions (entries 1–10 in Table 1), conversion of ar-himachalene 2 was almost complete in ca. 8 h.
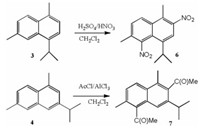
Structural assignment of 3 was confirmed by aromatic nitration with HNO3/H2SO4 (Scheme 2). Among various products, the dinitro derivative 6 was isolated with 65% yield after crystallization from EtOAc. The cadalenic structure 6 was confirmed by X-ray diffraction analysis of selected crystals, the data being identical to those previously reported (P1, triclinic space group, CCDC: 887079 [47].
|
Download:
|
| Scheme 2. Synthesis of dinitro-cadalene 6 and diacetyl-iso-cadalene 7 | |
Likewise, the structure of iso-cadalene 4 was confirmed by X-ray diffraction analysis of single crystals of the aromatic diacetylation product 7 obtained in 60% yield by treatment with AlCl3/AcCl in DCM at room temperature (P21/n monoclinic space group, CCDC: 1476547 [48].
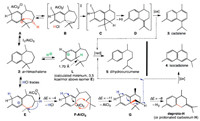
Although related rearrangements have ben previously reported, none of the proposed mechanistic processes are directly relevant to the present observations [49, 50]. A possible specific mechanism accounting for the formation of 3, 4 and 5 is therefore proposed (Scheme 3).
|
Download:
|
| Scheme 3. Proposed mechanism for the formation of cadalene 3, iso-cadalene 4 and dihydrocurcumene 5 from ar-himachalene 2. Indicated energy changes ΔE are in kcal/mol: they were calculated in the gas phase at the DFT level (without zero-point correction). For more detail, see Supporting information. | |
In DCM, the formation of cadalene 3 would start by electrophilic iodination of the less hindered aromatic CH site of 2 to the cyclohexadienyl cation intermediate A. Deprotonative rearrangement of A would then give the tricyclic spiro-cyclopropane C, via a transition state B of carbene-alkene type. Aromaticity recovery and steric relaxation would act as synergic driving forces for I–/H+ dissociation and cyclopropane ring opening to the exocyclic alkene D. Oxygen of air or I2 would finally play the role of more classical reagents (such as DDQ or chloranil) for oxidative aromatization of D to cadalene 3 [35-40].
In cyclohexane, traces of HCl, e.g., resulting from the reaction of traces of water with AlCl3, would trigger the reversible formation of the cyclohexadienylium salt E. Deprotonative cleavage of the benzylic C1-C11 bond of E would then give the spiro-cyclobutane G, via a transient cyclohexadiene-cyclopropane-carbenoid intermediate F-AlCl3 (stabilized form of the free carbene F: see exploratory DFT calculations in Supporting information, and references [51-53] for Al-NHC complexes). Under the synergic driving forces of steric relief and aromaticity recovery, dehydrogenation of G would then lead to the exocyclic alkene deproto-H or/via the corresponding tertiary carbocation H. After isomerization to the π-conjugated endocyclic alkene, the dihydronaphthalene bicycle would readily undergo oxidative aromatization to iso-cadalene 4, mediated by either air or carbenium L. This carbenoid route is also in line with the long-evidenced carbene intermediates in thermal rearrangements of polycyclic aromatics [54, 55], and in particular the related thermal rearrangement of azulenes to naphthalenes [56, 57].
Although steric relaxation of cadalene 3 to iso-cadalene 4 might be envisaged by thermal rearrangement under completely different conditions [54-57], the isolation of 3 in up to 76% yield allows ruling out thermodynamic control (calculation shows that 4 is 2.4 kcal/mol lower in energy than 3, Supporting information).
In default of AlCl3, the competing formation of dihydrocurcumene 5 can result from the opening of the cyclohexadienyl ring of E or its isomer L, playing the role of hydride acceptors allowing the parallel ultimate oxidation steps to 3 or 4 (Scheme 3). Noteworthy is the long benzylic C1-C11 distance (1.70 Å) in the calculated equilibrium geometry of L (Supporting information), which can thus be regarded as the intermediate of an ipso-substitution process by H+ [58]. The carbenium L would be reduced to 5 by serving as a co-oxidant in the conversion of D or H to 3 or 4, always formed simultaneously.
As a dramatic solvent effect, the use of cyclohexane instead of DCM is found to prevent the formation of cadalene 3, while favoring the formation of 4 and 5 (Table 1). This is accounted for by the lack of hetero-polar reactivity of iodine I2 and C—I bonds in a polar medium at the respective steps 2 → A and C → D.
A convenient synthesis of cadalene 3 and iso-cadalene 4 from a mixture of isomeric α-, β- and γ-himachalenes isolated from essential oil of Atlas cedar waste wood has thus been disclosed. The strategy involves two steps: after quantitative dehydrogenation of himachalenes, treatment of the resulting ar-himachalene 2 with I2 and/or AlCl3 affords 3 and 4 in proportions depending on the operating conditions. While the use of AlCl3 in the presence of I2 in DCM orientates the selectivity to cadalene 3 (isolated in up to 76% yield), the presence of AlCl3 in the absence of I2 in cyclohexane allows iso-cadalene 4 to be isolated in up to 66% yield. Last but not least, it is remarkable that in the absence of AlCl3 or I2 dihydrocurcumene 5 can form with up to 70% selectivity. Beyond preliminary DFT calculations of equilibrium structures just happening to not be in thermodynamic contradiction with the proposed mechanism (Supporting information), further claim would require to be confirmed or refined by calculations of transition states, which was out of the scope of this work. Nevertheless, the solvent- and Lewis acid-controlled versatility of the reaction outcome from the same cheap himachalene material is a typical illustration of the concept of dual roles design in organic synthesis, beyond general Green Chemistry principles [59].
In the future, the mechanism could also be refined by further identificationof sideproducts. Beyond the additional basic knowledge brought to the field of aromatic chemistry, the results open prospects for further industrial exploitation of the essential oil of Atlas cedar.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors are grateful to the Centro de Instrumentación Cientifica, Universidad de Granada and to Professor Rachid Chahboun Karimi for NMR experiments.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.008.
| [1] |
J. Gershenzon, N. Dudareva, Nat. Chem. Biol. 3 (2007) 408-414. DOI:10.1038/nchembio.2007.5 |
| [2] |
J. Zhou, Y. Zhang, Cell Cycle 7 (2008) 1360-1370. DOI:10.4161/cc.7.10.5953 |
| [3] |
C.T. Jordan, Cancer Cell 10 (2006) 253-254. DOI:10.1016/j.ccr.2006.09.010 |
| [4] |
B.T. Kawaraki, Prostrate 69 (2009) 827-837. |
| [5] |
N. Ravasio, F. Zaccheria, M. Guidotti, R. Psaro, Top. Catal. 27 (2004) 157-168. DOI:10.1023/B:TOCA.0000013550.28170.6a |
| [6] |
J.L.F. Monteiro, C.O. Veloso, Top. Catal. 27 (2004) 169-180. DOI:10.1023/B:TOCA.0000013551.99872.8d |
| [7] |
A. El Haib, A. Benharref, S. Parrès-Maynadié, et al., Tetrahedron Asymmetry 27 (2010) 1272-1277. |
| [8] |
E. Lassaba, A. Chekroun, A. Benharref, et al., Bull. Soc. Chim. Belg. 106 (1997) 281-288. |
| [9] |
A. Benharref, A. Chekroun, J.P. Lavergne, Bull. Soc. Chim. France 128 (1991) 738-741. |
| [10] |
A. Chiaroni, C. Riche, A. Benharref, E. Lassaba, A. Baouid, Acta Cryst. C 52 (1996) 2504-2507. DOI:10.1107/S0108270196005756 |
| [11] |
M. Loubidi, D. Agustin, A. Benharref, R.C.R. Poli, Chimie 17 (2014) 549-556. DOI:10.1016/j.crci.2014.01.023 |
| [12] |
A. El Haib, A. Benharref, S. Parrès-Maynadié, et al., Tetrahedron:Asymmetry 22 (2011) 101-108. DOI:10.1016/j.tetasy.2010.12.013 |
| [13] |
A. Chaudhary, P. Das, A. Mishra, Mol. Divers. 16 (2012) 357-366. DOI:10.1007/s11030-012-9372-3 |
| [14] |
A. Chaudhary, S. Sood, P. Das, et al., EXCLI J. 13 (2014) 1216-1225. |
| [15] |
R. Shankaranarayan, S.C. Bisarya, S. Dev, Tetrahedron 33 (1977) 1207-1210. DOI:10.1016/0040-4020(77)80416-X |
| [16] |
R.K. Boeckman Jr., D.M. Blum, B. Ganem, N. Halvey, Org. Synth. 58 (1978) 152-157. DOI:10.15227/orgsyn.058.0152 |
| [17] |
J. Daunis, R. Jacquier, H. Lopez, P. Viallefont, J. Chem. Res. (1983) o639-o649. |
| [18] |
B. Abouhamza, S. Allaoud, A. Karim, Molecules 6 (2001) M236. DOI:10.3390/M236 |
| [19] |
G.H. Jimenez-Alemana, T. Schöner, A.L. Montero-Alejo, W. Brandt, W. Boland, Arkivo (2012) 371-378. |
| [20] |
A. Chaudhary, S. Sood, P. Das, et al., EXCLI J. 13 (2014) 1216-1225. |
| [21] |
P. Teisseire, M. Plattier, Recherche 19 (1963) 153. |
| [22] |
J. Daunis, R. Jacquier, H. Lopez, O. Viallefont, J. Chem. Res. Synopses 2 (1987) 45. |
| [23] |
R. Alexander, R.I. Kagi, R.R. Singh, I.B. Sosrowidjojo, Org. Geochem. 21 (1994) 115-120. DOI:10.1016/0146-6380(94)90148-1 |
| [24] |
A. de O. Gomes, D. de A. Azevedo, J. Braz. Chem. Soc. 14 (2003) 358-368. DOI:10.1590/S0103-50532003000300004 |
| [25] |
M. Bordoloi, V.S. Shukla, S.C. Nath, R.P. Sharma, Phytochemistry 28 (1989) 2007-2037. DOI:10.1016/S0031-9422(00)97915-9 |
| [26] |
B.G.K. Van Aarssen, H.G. Cox, P. Hoogendoorn, J.W. de Leeuw, Geochem. Cosmochim. Acta 54 (1990) 3021-3031. DOI:10.1016/0016-7037(90)90119-6 |
| [27] |
L. Ruzicka, C.F. Seidel, Helv, Chim. Acta 5 (1922) 369-375. DOI:10.1002/hlca.19220050307 |
| [28] |
N.S. Gill, F. Lions, J. Am. Chem. Soc. 72 (1950) 3468-3469. DOI:10.1021/ja01164a039 |
| [29] |
W.S. Johnson, A. Russell Jones, J. Am. Chem. Soc. 69 (1947) 792-794. DOI:10.1021/ja01196a014 |
| [30] |
E. de Barry Barnett, W. Cook, J. Chem. Soc. 22 (1933) 22-24. |
| [31] |
R.P. Linstead, K.O.A. Michaelis, S.L.S. Thomas, J. Chem. Soc. (1940) 1139-1147. DOI:10.1039/jr9400001139 |
| [32] |
J.H. Kim, H.J. Lee, G.S. Kim, et al., Cancer Lett. 213 (2004) 139-145. DOI:10.1016/j.canlet.2004.03.049 |
| [33] |
A.K. Banerjee, P.S. Poon, Arkivoc 13 (2009) 108-115. DOI:10.1016/S0065-3160(08)00006-3 |
| [34] |
H.Y. Lee, J.T. Kwon, M. Koh, M.H. Cho, S.B. Park, Bioorg. Med. Chem. Lett. 17 (2007) 6335-6339. DOI:10.1016/j.bmcl.2007.08.071 |
| [35] |
T.C. Joseph, S. Dev, Tetrahedron Lett. 2 (1961) 216-222. DOI:10.1016/S0040-4039(01)99234-2 |
| [36] |
B. Josshi, R. Seshadri, K. Charkravarti, S. Bhattacharyya, Tetrahedron 20 (1964) 2911-2919. DOI:10.1016/S0040-4020(01)98512-6 |
| [37] |
T.C. Joseph, S. Dev, Tetrahedron 24 (1968) 3809-3827. DOI:10.1016/S0040-4020(01)92589-X |
| [38] |
R.C. Pandey, S. Dev, Tetrahedron 24 (1968) 3829-3839. DOI:10.1016/S0040-4020(01)92590-6 |
| [39] |
J. Daunis, R. Jacquier, H. Lopez, P.J. Viallefont, Med. Chem. Res. (1981) o639-o649. |
| [40] |
B. Abouhamza, S. Allaoud, A. Karim, Molecules 6 (2001) M236. DOI:10.3390/M236 |
| [41] |
R.L. Cobb, US patent 4551573, 1985.
|
| [42] |
R.D. Batt, S.N. Slater, J. Chem. Soc. (1949) 838-842. |
| [43] |
M.A. Al-Qudah, A.M. Al-Ghoul, I.N. Trawenh, et al., J. Biol. Active Prod. Nat. 4 (2014) 52-61. |
| [44] |
S.V. Nampoothiri, R.M. Philip, S. Kankangi, C.R. Kiran, A.N. Menon, J. Essent, Oil. Bear. Plants 18 (2015) 1051-1058. DOI:10.1080/0972060X.2014.908746 |
| [45] |
S. Sandeep, A. Kuanara, A. Akbar, et al., Ind. Crops Prod. 85 (2016) 229-240. DOI:10.1016/j.indcrop.2016.03.007 |
| [46] |
H. Xiang, L. Zhang, Z. Yang, et al., Ind. Crops Prod.2017 108 (2017) 6. DOI:10.1016/j.indcrop.2017.05.058 |
| [47] |
M. Chakkar, N. Oughris, A. Benharref, J.C. Daran, M. Berraho, Acta Cryst. Sect. E 68 (2012) o1893. DOI:10.1107/S1600536812021514 |
| [48] |
A. Benharref, A. Oukhrib, M. Ait Elhad, et al., IUCr 1 (2016) x160703. DOI:10.1107/S2414314616007033 |
| [49] |
G. Frater, U. Muller, Chem. Commun. (1988) 1198-1200. |
| [50] |
A. Scott, S.A. Snyder, D.S. Treitler, A.P. Brucks, J. Am. Chem. Soc. (2010) 14303-14314. |
| [51] |
A. Stasch, S. Singh, H.W. Roesky, M. Noltemeyer, H.G. Schmidt, Eur. J. Inorg. Chem. (2004) 4052-4055. DOI:10.1002/ejic.200400247 |
| [52] |
V. Nestereov, D. Reiter, P. Bag, et al., Chem. Rev. 118 (2018) 9678-9842. DOI:10.1021/acs.chemrev.8b00079 |
| [53] |
C.H. Wang, Y.F. Lin, H.C. Tseng, et al., Eur. J. Inorg. Chem. (2018) 2232-2236. |
| [54] |
L.T. Scott, Acc. Chem. Res. 15 (1982) 52-58. DOI:10.1021/ar00074a004 |
| [55] |
L.T. Scott, M.M. Hashemi, T.H. Schultz, M.B. Wallace, J. Am. Chem. Soc. 113 (1991) 9692-9693. DOI:10.1021/ja00025a055 |
| [56] |
L.T. Scott, J. Org. Chem. 49 (1984) 3022-3024. DOI:10.1021/jo00190a031 |
| [57] |
C. Perrin, G.A. Skinner, J. Am. Chem. Soc. 93 (1971) 3389. DOI:10.1021/ja00743a015 |
| [58] |
J.R. Martyn, Ipso-Aromatic Substitution Ph. D. thesis, University of Canterbury, 1985, and references therein.
|
| [59] |
(a) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138; (b) K.J. Liu, J.H. Deng, J. Yang, et al., Green Chem. 22 (2020) 433-438; (c) C. Wu, X. Xin, Z.M. Fu, et al., Green Chem. 19 (2017) 1983-1989. |
 2020, Vol. 31
2020, Vol. 31 


