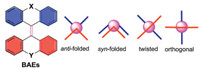
Smart stimuli-responsive materials have received much attention in digital technology, sensors, material biology and biomedical applications during the past few years [1, 2]. The responsive materials can be categorized on the basis of their responsive stimuli and behaviors, and various responsive materials, such as vapochromic, piezoelectric, electrostrictive, magnetostrictive, mechanochromic, photo-responsive, thermo-responsive, pH-sensitive materials, have been well developed [3-13]. Mechanochromic molecules refer to the force-responsive molecules that exhibit an optical response in reaction to the application of an external force stimulus [14]. Mechanochromic molecules have been extensively studied in the field of supramolecular chemistry, polymeric mechanochromic materials, mechanochemical transduction, stress sensing and so on [15, 16]. Among the various reported mechanochromic molecules, the mechanochromism of bistricyclic aromatic enes (BAEs) was first to be realized and well investigated [17]. BAEs are defined as overcrowded alkenes consisting of two tricyclic aromatic units that are connected through a C—C double bond (Fig. 1). The synthesis of BAEs and the discovery of their chromism could be traced back to more than 100 years ago [18-20]. The chromism of BAEs could be triggered by various stimuli including thermal, mechanical, electrochemical, or optical stimuli, resulting in their corresponding thermo-, mechano-, electro-, and photochromic phenomenon, respectively [21-23]. The chromism of BAEs is believed to be associated with their conformational change from a folded conformer to a twisted conformer (Fig. 1) [24-27]. However, the chromism in BAEs is still an enigma as the exact mechanism in such process remains controversial. In fact, there are many rationales that have been proposed to explain the chromism in BAEs such as the aggregation–disaggregation equilibrium, zwitterionic formation, solid-state effects, double-chair conformation, diradicals formation and so on [28]. The paramagnetic diradical formation in connection with the chromism was initial suspected, however, was later refuted because no solid evidence supported the increased susceptibility [29, 30]. Nevertheless, recent study disclosed that some overcrowded molecules related to BAEs were able to be populated to the excited triplet diradicals [31, 32].
|
Download:
|
| Fig. 1. General structure of bistricyclic aromatic enes and their four conformations. | |
In this paper, an unexpected bistricyclic aromatic ene AF was obtained when the precursor of AF-diols was reduced in the presence of tin(Ⅱ) chloride (SnCl2). The formation of such unexpected AF was likely due to a carbocation induced 1, 2-migration during the SnCl2-mediated reductive aromatization reaction. The crystallographic analysis revealed that AF was overcrowded and forced to adopt a folded structural conformation. Interestingly, AF exhibited excellent mechanochromism and thermochromism property upon being stimulated to its twisted conformation. Preliminary results also indicated that the twisted conformation of AF could be thermally populated to a triplet excited state that formed trace diradical species.
The synthesis of AF is very straightforward and starts from the reported diols intermediate of AF-diols that was prepared from 9, 10-dibromoanthracene and fluoren-9-one under standard conditions (Scheme 1) [33]. Synthesis and characterization of compound AF is deposited in Supporting information. The SnCl2-mediated reductive aromatization reaction was carried out to reduce AF-diols. Our initial propose was to synthesize the target molecule AF', which was very recently realized by Campos and coworkers [34]. The related molecule was reported to have a thermally accessible open-shell ground-state configuration and interesting aggregation induced emission [34-37]. In contrast, it was found that the dichloromethane (DCM) solution of AF-diols quickly changed into red without any emission upon adding SnCl2. Finally, the main product of AF was isolated as a pale solid in 65% yield.

|
Download:
|
| Scheme 1. Synthetic route to AF and the plausible mechanism. | |
The obtained AF was first characterized by 1H and 13C NMR spectrometry (Fig. 2). Surprisingly, the 1H NMR spectrum showed very complicated proton signals that obviously cannot be assigned to the expected AF' with highly symmetric structure [34]. Moreover, the 13C NMR spectrum showed a resonance signal at 195.4 ppm, indicating the existence of C=O group. Fortunately, the actual structure of AF was finally unambiguously determined by X-ray crystallographic analysis (vide infra). A hypothetical reactio mechanism was proposed in Scheme 1 based on some similar documented results, as well as our chemical intuition [38, 39]. The formation of the unexpected AF was likely caused by a carbocation induced 1, 2-rearrangement during the SnCl2-mediated reductive aromatization reaction. Interestingly, during the revision of our manuscript, a similar work [40] reported a highly strained molecule via the similar synthetic protocol. However, such unexpected reaction was not observed in this report. This is probably because the resultant spiro ketone with seven membered ring is not favourable during this 1, 2-rearrangement reaction.

|
Download:
|
| Fig. 2. The 1H and 13C NMR spectra of AF recorded in 1, 1, 2, 2-tetrachloroethane-d2. | |
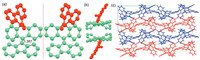
The slow diffusion of methanol into the red DCM solution of AF resulted in the pale single crystal that was suitable for X-ray crystallographic analysis. The crystallographic data of AF (CCDC: 1969049) can be obtained free of charge from the Cambridge Crystallographic Data Centre. As shown in Fig. 3, the skeleton of AF was consisted of a bistricyclic aromatic ene unit (highlight in green color) and 10H-phenanthren-9-one unit (highlight in red color). The length of the central C—C double bond was determined to be ≈1.36 Å, which is a common result as the central double bond of all BAEs is reported to be short in their folded conformations [17-27]. Interestingly, AF molecule as a whole was highly of all BAEs is reported to be short in their folded conformations [17-27]. Interestingly, AF molecule as a whole was highly 10H-phenanthren-9-one unit, thus resulting in a spiro configuration. The spiro AF possessed a racemic isomeric pair in its solid state, which could not be separated because of the small rotation barrier. The two isomers were aligned alternately and packed into a three-dimensional array through intermolecular CH/π interaction.
|
Download:
|
| Fig. 3. X-ray crystallographic structure of AF: (a) top view, (b) side view and (c) 3D packing. CH/π interactions are highlighted in pink. | |
To our delight, AF featured very interesting chromism property. For example, the color of AF in solid state was pale while its DCM solution showed high-contrast red color. At first, we inferred that such solvatochromic phenomenon mightbe related to its intrinsic zwitterionic character [28]. Thus, the absorption spectra of AF have been investigated in different solvents (Fig. S6 in Supporting informaition). However, it was found that the absorption profile of AF was almost independent of the solvent polarity, illustrating that such chromism is not originated from its zwitterionic character. In addition, the chromism of AF could also be triggered bymechanical andthermal stimuli. When the pale-colored solid AF was ground with anagate mortar and pestle (Fig. 4a), its color changed immediately from pale to red. This color switching behavior is basically based on the conformational change of AF, i.e., AF switched from the folded conformation to the twisted one during grinding. Notably, such colorchange can be reversed through exposure to DCM vapor. Likewise, the toluene solution of AF revealed thermochromic phenomenon when heating to 100 ℃ (Fig. 4b), although the phenomenon was not so obvious compared to the mechanochromism. Through the comparison and analysis, it was implied that mechanical and thermal stimuli could both produce the twisted conformation species that exhibited red color. Notably, it is found that AF exhibited very weak photoluminescence both in solution and solid state (Figs. S11–S13 in Supporting information), probably due to their folded/twisted molecular geometries.

|
Download:
|
| Fig. 4. (a, b) Chromism properties of AF. (c) Variable-temperatureEPR spectrum of AF. (d) Conformation inversion and thermally populated excited state of AF. | |
To gain more insight into the interesting chromism property, the NMR and electron paramagnetic resonance (EPR) spectra of AF before and after stimuli have been investigated. The variabletemperature 1H NMR spectrum of AF revealed no significant change in line broadening or splitting (Fig. S7 in Supporting information). Similarly, the solid NMR spectrum of AF before and after grinding almost presented the same profile (Figs. S8 and S9 in Supporting information). Moreover, the negligible EPR response of AF almost remained no change before and after grinding (Fig. S10). It seems that such chromism behaviour has nothing to do with the radical formation. However, when we carefully checked the temperature-dependent EPR of solid AF, a very weak single-line EPR spectrum was observed. With the increase of temperature, the intensity of EPR signal increased (Fig. 4c), thus suggesting the formation of trace amount of excited triplet diradicals [32]. It is well known that EPR is more sensitive than NMR regarding to the paramagnetic species. Our preliminary results implied that the twisted conformation of AF could be thermally populated to a triplet excited state and form trace diradical specie, which was detectable in EPR (Fig. 4d). The trace amount paramagnetic species were not enough to affect the relaxation rates (e.g., T2 relaxation time) [41, 42], thus expressing the detectable sharp NMR spectra.
In conclusion, we herein present the synthesis and characterization of an unexpected mechanochromic bistricyclic aromatic ene AF, which was coincidentally synthesized in a SnCl2-mediated reductive aromatization reaction in a good yield most likely through a carbocation induced 1, 2-migration. The crystallographic analysis disclosed the real molecular structure of AF consisting of a bistricyclic aromatic ene linked with a 10H-phenanthren-9-one unit. In the single crystal, AF adopted a folded conformation and spiro configuration with a pair of inseparable racemic isomers. Interestingly, AF exhibited reversible high-contrast mechanochromism and thermochromism between pale and red color. The red color appearance was assigned to the twisted conformation, which was realized through mechanical and thermal stimuli. Furthermore, the EPR results may give some hints that AF in its twisted conformation state could be further populated to the excited triplet diradicals. This study would hopefully help to understand the enigma of thermochromismin BAEsfrom a different view.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsH.-B. Yang thanks Innovation Program of Shanghai Municipal Education Commission (No. 2019-01-07-00-05-E00012), Program for Changjiang Scholars and Innovative Research Team in University for financial support. X. Shi acknowledges the financial supports sponsored by Shanghai Sailing Program (No. 19YF1412900) and the Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.02.010.
| [1] |
R. Yerushalmi, A. Scherz, M.E. van der Boom, H. Kraatz, J. Mater. Chem. 15 (2005) 4480-4487. DOI:10.1039/b505212b |
| [2] |
P. Theato, B. Sumerlin, R.O'Reilly, T. EppsIII, Chem.Soc.Rev. 42 (2013) 7055-7056. DOI:10.1039/c3cs90057f |
| [3] |
T.T. Cao, X.Y. Yao, J. Zhang, et al., Chin. Chem. Lett. 26 (2015) 867-871. DOI:10.1016/j.cclet.2015.01.032 |
| [4] |
J. Wu, Y. Cheng, J. Lan, et al., J. Am. Chem. Soc. 138 (2016) 12803-12813. DOI:10.1021/jacs.6b03890 |
| [5] |
Y. Wang, X. Tan, Y.M. Zhang, et al., J. Am. Chem. Soc. 137 (2015) 931-939. DOI:10.1021/ja511499p |
| [6] |
J.F. Xu, Y.Z. Chen, D. Wu, et al., Angew. Chem. Int. Ed. 52 (2013) 9738-9742. DOI:10.1002/anie.201303496 |
| [7] |
X. Yan, J.F. Xu, T.R. Cook, et al., Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 8717-8722. DOI:10.1073/pnas.1408620111 |
| [8] |
J. Yi, W. Liang, X. Wei, et al., Chin. Chem. Lett. 29 (2018) 87-90. DOI:10.1016/j.cclet.2017.05.004 |
| [9] |
J. Yao, W. Wu, W. Ling, et al., Angew. Chem. Int. Ed. 56 (2017) 6869-6873. DOI:10.1002/anie.201702542 |
| [10] |
X. Ma, H. Tian, Acc. Chem. Res. 47 (2014) 1971-1981. DOI:10.1021/ar500033n |
| [11] |
Z. Fu, K. Wang, B. Zou, Chin. Chem. Lett. 30 (2019) 1883-1894. DOI:10.1016/j.cclet.2019.08.041 |
| [12] |
X. Yao, T. Li, J. Wang, et al., Adv. Opt. Mater. 4 (2016) 1322-1349. DOI:10.1002/adom.201600281 |
| [13] |
B. Jiang, J. Zhang, J.Q. Ma, et al., J. Am. Chem. Soc. 138 (2016) 738-741. DOI:10.1021/jacs.5b11409 |
| [14] |
D.J.Fisher, Mechanochromism, Materials Research Forum, LLC, Millersville, 2019.
|
| [15] |
J. Li, C. Nagamani, J.S. Moore, Acc. Chem. Res. 48 (2015) 2181-2190. DOI:10.1021/acs.accounts.5b00184 |
| [16] |
C. Calvino, L. Neumann, C. Weder, S. Schrettl, J. Polym, Sci. Part A:Polym. Chem. 55 (2017) 640-652. DOI:10.1002/pola.28445 |
| [17] |
P.U. Biedermann, I. Agranat, Top. Curr. Chem. 350 (2014) 177-277. |
| [18] |
H. Meyer, Ber. Dtsch. Chem. Ges. B 42 (1909) 143-145. DOI:10.1002/cber.19090420118 |
| [19] |
H. Meyer, Chemistry 30 (1909) 165-177. |
| [20] |
J.H. Day, Chem. Rev. 63 (1963) 65-80. DOI:10.1021/cr60221a005 |
| [21] |
P.U. Biedermann, J.J. Stezowski, I. Agranat, Eur. J. Org. Chem. (2001) 15-34. DOI:10.1002/chin.200208299 |
| [22] |
A. Levy, S. Pogodin, S. Cohen, I. Agranat, Eur. J. Org. Chem. (2007) 5198-5211. DOI:10.1002/ejoc.200790082 |
| [23] |
S. Pogodin, M.R. Suissa, A. Levy, et al., Eur. J. Org. Chem. (2008) 2887-2894. |
| [24] |
Y. Matsuo, Y. Wang, H. Ueno, et al., Angew. Chem. Int. Ed. 58 (2019) 8762-8767. DOI:10.1002/anie.201902636 |
| [25] |
T. Suzuki, H. Okada, T. Nakagawa, et al., Chem. Sci. 9 (2018) 475-482. DOI:10.1039/C7SC03567E |
| [26] |
M. Filatov, ChemPhysChem 12 (2011) 3348-3353. DOI:10.1002/cphc.201100444 |
| [27] |
Y. Hirao, Y. Hamamoto, N. Nagamachi, T. Kubo, Phys. Chem. Chem. Phys. 21 (2019) 12209-12216. DOI:10.1039/C9CP01836K |
| [28] |
P.U. Biedermann, J.J. Stezowski, I. Agranat, Chem. Eur. J. 12 (2006) 3345-3354. DOI:10.1002/chem.200501118 |
| [29] |
W.G. Nielsen, G.K. Fraenkel, J. Chem. Phys. 21 (1953) 1619-1624. |
| [30] |
G. KortFm, K.W. Koch, Chem. Ber. 100 (1967) 1515-1520. DOI:10.1002/cber.19671000517 |
| [31] |
Z. Zeng, Y. Sung, N. Bao, et al., J. Am. Chem. Soc. 134 (2012) 14513-14525. DOI:10.1021/ja3050579 |
| [32] |
C. Wentrup, M.J. Regimbald-Krnel, D. Müller, P. Comba, Angew. Chem. Int. Ed. 55 (2016) 14600-14605. DOI:10.1002/anie.201607415 |
| [33] |
A. Wierig, W. Seichter, I. Thondorf, E. Weber, Supramol.Chem. 24 (2012) 713-725. DOI:10.1080/10610278.2012.701301 |
| [34] |
X. Yin, J.Z. Low, K.J. Fallon, et al., Chem. Sci. 10 (2019) 10733-10739. DOI:10.1039/C9SC04096J |
| [35] |
Y. Cai, L. Du, K. Samedov, et al., Chem. Sci. 9 (2018) 4662-4670. DOI:10.1039/C8SC01170B |
| [36] |
S. Li, M. Gao, S. Wang, et al., Chem. Commun. 53 (2017) 4795-4798. DOI:10.1039/C7CC01602F |
| [37] |
J. Mei, N.C. Leung, R.T.K. Kwok, et al., Chem. Rev. 115 (2015) 11718-11940. DOI:10.1021/acs.chemrev.5b00263 |
| [38] |
F. Ciminale, L. Lopez, G. Mele, Tetrahedron 44 (1994) 12685-12696. DOI:10.1016/s0040-4020(01)89401-1 |
| [39] |
L. Lopez, G.M. Farinola, A. Nacci, S. Sportelli, Tetrahedron 54 (1998) 6939-6946. DOI:10.1016/S0040-4020(98)00375-5 |
| [40] |
T. Nishiuchi, R. Ito, E. Stratmann, T. Kubo, J. Org. Chem. 85 (2020) 179-186. DOI:10.1021/acs.joc.9b02432 |
| [41] |
L.J. Berliner, J. Reuben, Biological Magnetic Resonance, Vol. 12, Plenum, New York, 1993.
|
| [42] |
E.D. Becker, High Resolution NMR: Theory and Chemical Applications, 3rd ed., Academic Press, New York, 1999.
|
 2020, Vol. 31
2020, Vol. 31 

