b Research Institute of Sun Yat-sen University in Shenzhen, Shenzhen 518057, China
As the largest organ of human body, skin is vulnerable to various pathogenic or injurious factors, leading to skin trauma [1]. The wound healing is a complicated process and could be divided into four overlapped phases: hemostasis, inflammation, proliferation and tissue remodeling [2]. However, this orderly healing process can be easily disturbed and limited in the inflammatory phase by many inherent or external factors (e.g., overproduction of reactive oxygen species (ROS), hyperglycemia and bacterial infection), resulting in the chronic wound healing [3, 4]. It is still a worldwide challenge to achieve the effective healing of chronic skin trauma.
Wound dressing has developed into the main treatment strategy for skin trauma at present due to the pathological characteristics of skin trauma. At current stage, various kinds of wound dressing have been designed and fabricated, such as hydrogel dressing [5, 6], nano-fiber dressing [7, 8], foam dressing [9] and hydrocolloid dressing [10]. Among them, hydrogel dressing has caught significant attentions due to its flexible physicochemical properties and the ability to provide moist environment and simulate extracellular matrix [11]. Especially, the in situ formed injectable hydrogel is of particular interest as it could be easily applied on irregular wound sites and prevent the invasion of pathogen [12].
Scutellaria baicalensis Georgi, known as one of the three yellow herbs in traditional Chinese medicine, was frequently utilized for the treatment of chronic trauma in ancient China [13]. Modern medical researches indicate that baicalin (Fig. S1 in Suppoting information), the major component extracted from Scutellaria baicalensis Georg, possesses multiple pharmacological activities [14], such as anti-oxidant, bacteriostasis, diuresis and antiinflammatory, and has already been widely used for cancer therapy [15]. Both therapy practice and biological activities of baicalin highlight its potential application for chronic trauma therapy. However, the poor solubility and lack of suitable vehicles limit its further therapy applications [16,17].
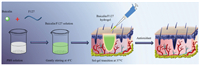
In the present work, we proposed a simple and robust strategy to enhance the solubility of baicalin and prepared baicalin/ Pluronic® F-127 (F127) injectable hydrogel to study its potential wound healing application, mainly focusing on antioxidant activity (Fig. 1). First, we utilized PBS solution as solvent to realize the enhanced solubility of baicalin and the preparation of baicalin/ F127 hydrogel. Then, the obtained baicalin/F127 hydrogel was investigated using different tests including Fourier transform infrared spectroscopy (FTIR), rheological testing, releasing behavior testing and anti-oxidant assay. Furthermore, the in vivo assessment of baicalin/F127 hydrogel was carried out to evaluate its potential application for chronic wound therapy.
|
Download:
|
| Fig. 1. The illustration of the preparation of baicalin/F127 hydrogel and its application for wound healing. | |
To improve the solubility of baicalin, a convenient method was employed here by using PBS solution as solvent. As Fig. 2a showed, baicalin formed a turbid suspension in deionized water (1.5 mg/mL), revealing its poor solubility (left), however, transparent and clean solution could be obtained after using PBS solution as solvent with the same concentration of baicalin (right). The enhanced solubility of baicalin largely cleaned its application obstacles for wound healing.

|
Download:
|
| Fig. 2. The preparation and characterization of baicalin/F127 hydrogel. (a) The photograph of baicalin dissolved in deionized water (left) and PBS solution (right). (b) ATR-FTIR spectra of baicalin, F127 hydrogel and different baicalin/F127 hydrogels. (c) Release profile of baicalin from baicalin/F127 hydrogels in PBS solution. | |
In this study, Pluronic® F-127 (F127) was selected as hydrogel matrix to realize the delivery of baicalin in wound site. The FTIR spectrum demonstrated the composition of baicalin/F127 hydrogel. As Fig. 2b showed, absorption bond of -CH3 (2, 880 cm-1) and C-O-C (1, 110 cm-1) [18] were detected in hydrogel samples, indicating the presence of F127. As the content of baicalin in hydrogels was very little, its characteristic absorption peak (-COOH (1, 745 cm-1), C-O-C (1, 076 cm-1) [19]) was obscure. F127, a block copolymer composed of polyethylene oxide (PEO) and polyoxypropylene (PPO), performed temperature-dependent solgel transition [20]. At low temperature, injectable and homogenous baicalin/F127 solutions could be obtained by PBS solvent, but at around body temperature (37 ℃), baicalin/F127 solutions transformed into baicalin/F127 hydrogels (Fig. S2 in Supporting information). Rheological testing demonstrated such sol-gel transition. Fig. S3 (Supporting information) performed the changes in storage modulus (G') and loss modulus (G") of different samples as a function of temperature. The gel point was generally defined as the intersection point of G' and G". The G' and G" of sol state maintained a low value and increased sharply at around gel point, indicating the sol-gel transition. After the finish of transition, G' and G" of gel state maintained a relative high value. The addition of baicalin did not change the temperature sensitivity of F127 solution (hydrogel), however, the gel temperature of baicalin/F127 solutions increased by about 4 ℃ compared with pure F127 hydrogel.
The release profile of baicalin from F127 hydrogel was depicted in Fig. 2C. It was clearly observed that initial drug loading with concentrations range from 0.5 mg/mL to 1.5 mg/mL did not remarkably affect the release characteristics of baicalin from F127 hydrogel. A rapid release could be achieved in the initial 8 h with more than 70% cumulative release, followed by a gradual release until 24 h. In the early phase of wound healing, the rapid release of baicalin was desired as it could reduce the reactive oxygen species (ROS) produced during the inflammatory to accelerate wound healing. Three kinetic models were employed to analysis the release kinetic of baicalin/F127 hydrogels. As Table S2 (Supporting information) showed, the first order model was best fitted with the release behavior of baicalin/F127 hydrogels.
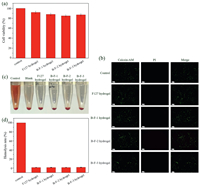
Biocompatibility is one of vital factors for the clinical application of hydrogel dressings. As MTT assay results showed (Fig. 3a), all of the cell viability of NIH 3T3 cells treated with different hydrogel extracts were more than 80%, indicating excellent biocompatibility. Besides, the living/dead staining assay was further used to verify the biocompatibility. Normal cells could be stained with calcein-AM and present green morphology, while dead cells presented red morphology after being stained with PI. As Fig. 3b indicated, all of different hydrogel samples showed good biocompatibility. In addition, the hemolysis activity of hydrogel samples was evaluated by direct contact method. As Fig. 3c presented, the hemolysis activity of hydrogel samples was similar to that of blank sample. The supernatants were transparent and colorless, indicating almost no damage of red cells. The quantitative analysis of hemolysis rate of hydrogel samples was also carried out (Fig. 3d). The hemolysis percentage of baicalin/F127 hydrogels was far less than 5% (maximum limit [21]), demonstrating excellent blood biocompatibility. The good cytocompatibility of baicalin/F127 hydrogels provided a guarantee for further application of skin wound treatment.
|
Download:
|
| Fig. 3. The biocompatibility of baicalin/F127 hydrogels. (a) The cell viability of NIH 3T3 cells treated with different baicalin/F127 hydrogel samples detected by MTT assay. (b) Microscopy images of NIH 3T3cells stained with calcein-AM and PI after co-culturing with different hydrogel samples. The scale bar is 100 μm. (c) Photograph of RBCs after incubation with different hydrogel samples. (d) Hemolysis ratio analysis of RBCs after incubation with different hydrogel samples. | |
In the process of wound repairing, antioxidant capacity is an important factor [22]. During the inflammation phase, in order to protect against invading pathogens and send intracellular signaling, low level of reactive oxygen species (ROS) was produced. However, the overproduction of ROS could cause damage to DNA and protein, leading to chronic wound healing [23]. In this work, we attempted to investigate the antioxidant activity of baicalin/ F127 hydrogels. DPPH was a kind of free radicals and could be neutralized by antioxidant agent, leading to the change of the solution color. As the DPPH assay results (Fig. 4a) showed, contrast to the F127 hydrogel, baicalin/F127 hydrogels performed significant DPPH scavenging activity. The higher the baicalin content was, the stronger the DPPH scavenging activity of baicalin/F127 hydrogel possessed, demonstrating the fact that the presence of baicalin in hydrogel was responsible for its antioxidant activity.

|
Download:
|
| Fig. 4. The antioxidant activity of baicalin/F127 hydrogel on wound healing. (a) The DPPH scavenging efficiency of baicalin/F127 hydrogels. (b) The ROS level detected by DCFH-DA staining after 24 h incubation. The scale bar is 100 mm. (c) The cell viability of NIH 3T3 cells treated with different hydrogel samples after the addition of H2O2. (d) The photos of full thickness excise wounds treated by B-F-3 hydrogel, F127 hydrogel and 3 M Tegaderm (control) at different time. (e) The closure rate of wounds at different time. *P < 0.05, **P < 0.01, ***P < 0.001. | |
Furthermore, NIH 3T3 cells were used to evaluate the cytoprotective activityof baicalin/F127 hydrogels against ROS.DCFH-DA had no fluorescence and could freely pass through the cell membrane. It was hydrolyzed by esterase in the cell to produce DCFH and oxidized by ROS to produce fluorescent DCF. As shown in Fig. 4b, the addition of H2O2 resulted in the production of large amount of ROS in cells. TheROS could be remarkably cleaned with the treatmentof baicalin/ F127 hydrogels, proving the cytoprotective activity of baicalin/F127 hydrogelsagainstROS. Besides, MTTassay results also confirmed this characteristic. As presented in Fig. 4c, cell viability of NIH 3T3 cells was about 50% after the addition of H2O2. With the treatment of baicalin/F127hydrogels, the cellviabilitywas significantlyenhanced, indicating the vital role of baicalin in hydrogel for its cytoprotective effect. As the ROS in cells could be almost completely cleaned by all baicalin/F127 hydrogel samples (Fig. 4b), there was no significant difference in the viabilityof cells treated with differentbaicalin/F127 hydrogel samples.
For wound healing measurement, B-F-3 hydrogel was selected as test group as its better antioxidant property. As Figs. 4d and e showed, B-F-3 hydrogel could significantly accelerate wound healing compared with control group. It was observed that after 10 days of treatment, the wound aero was about 18% for B-F-3 hydrogel, whereas the control group and F127 hydrogel group maintained 60% and 45% unclosed area, respectively. After 15 days of treatment, almost completely wound closure was observed for B-F-3 hydrogel, however, control group and F127 hydrogel group still remained about 45% and 15% unclosed wound aero, respectively. The enhanced wound healing could be attributed to the antioxidant activity of B-F-3 hydrogel. In the following work, we will carry on evaluating the in vivo results by histological analysis, and explain in detail the mechanism of baicalin/F127 hydrogel enhancing wound healing.
In conclusion, we used PBS solution as solvent to enhance the solubility of baicalin, greatly overcame its application obstacles for skin wound treatment. Furthermore, F127 was employed as hydrogel matrix to carry on baicalin to prepare injectable baicalin/F127 hydrogels. This novel injectable hydrogel could realize sol-gel transition at wound site and exhibit flexible antioxidant activity. The in vivo experiment demonstrated that this baicalin/F127 hydrogel significantly accelerate wound healing compared with F127 hydrogel and control group treated by commercial dressing (3 M Tegaderm), indicating promising potential in skin trauma treatment. In the future research, we will further study the mechanism of this kind of hydrogel improving wound healing by tissue histology and immunohistochemistry.
Declaration of competing interestThe authors confirm that no conflicts of interest exist regarding the content of this article.
AcknowledgmentsThis work was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2018ZX10301402), International Cooperation and Exchange of the National Natural Science Foundation of China (No. 51820105004), Science and Technology Program of Guangzhou (No. 201707010094), Guangdong Innovative and Entrepreneurial Research Team Program (Nos. 2013S086 and 2016ZT06S029), and Science and Technology Planning Project of Shenzhen (Nos. JCYJ20170307141438157 and JCYJ20180307163534533), Fundamental Research Funds for the Central Universities (No. 19lgpy209).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.005.
| [1] |
M. Rodrigues, N. Kosaric, C.A. Bonham, G.C. Gurtner, et al., Physiol. Rev. 99 (2017) 665-706. |
| [2] |
E.N. Arwert, E. Hoste, F.M. Watt, Nat. Rev. Cancer 12 (2012) 170-180. DOI:10.1038/nrc3217 |
| [3] |
S. Barrientos, O. Stojadinovic, M.S. Golinko, H. Brem, M. Tomic-Canic, Wound Repair Regen. 16 (2008) 585-601. DOI:10.1111/j.1524-475X.2008.00410.x |
| [4] |
W. Ma, S.N. Sha, P.L Chen, Adv. Healthcare Mater 9 (2020) e1901100. DOI:10.1002/adhm.201901100 |
| [5] |
Z. Bao, C. Xian, Q. Yuan, G. Liu, J Wu, Adv. Healthcare Mate 8 (2019) e1900670. DOI:10.1002/adhm.201900670 |
| [6] |
H. Fang, J. Wang, L. Li, et al., Chem. Eng. J. 365 (2019) 153-164. DOI:10.1016/j.cej.2019.02.030 |
| [7] |
J. He, Y. Liang, M. Shi, B Guo, Chem. Eng. J 385 (2019) 123464. |
| [8] |
K.A. Rieger, N.P. Birch, J.D. Schiffman, J. Mater. Chem. B 1 (2013) 4531-4541. |
| [9] |
D.G. Pyun, H.S. Yoon, H.Y. Chung, et al., Mater. Chem. B 3 (2015) 7752-7763. DOI:10.1039/C5TB00995B |
| [10] |
J.P. Draye, B. Delaey, A. van de Voorde, et al., Biomaterials 19 (1998) 1677-1687. DOI:10.1016/S0142-9612(98)00049-0 |
| [11] |
A.H. Morris, H. Lee, H. Xing, et al., ACSAppl.Mater.Interfaces 10 (2018) 41892-41901. DOI:10.1021/acsami.8b08920 |
| [12] |
J. Xiao, S. Chen, Y. Ji, H.F. Zhang, G.A Ameer, Adv. Funct. Mater 27 (2016) 1604872. |
| [13] |
H. Li, Y. Jiang, F. Chen, J. Chromatogr. B 812 (2004) 277-290. DOI:10.1016/S1570-0232(04)00545-8 |
| [14] |
Z. Gao, K. Huang, X. Yang, H. Xu, Biochim. Biophys. Acta 1472 (1999) 643-650. DOI:10.1016/S0304-4165(99)00152-X |
| [15] |
A.C. Scheck, K. Perry, N.C. Hank, W.D. Clark, BMC Complement. Altern. Med. 6 (2006) 27-36. DOI:10.1186/1472-6882-6-27 |
| [16] |
T. Krakauer, B.Q. Li, H.A. Young, FEBS Lett. 500 (2001) 52-55. DOI:10.1016/S0014-5793(01)02584-4 |
| [17] |
Q. Chen, Y. Yang, X. Lin, et al., Chem. Commun. 54 (2018) 5369-5372. DOI:10.1039/C8CC02791A |
| [18] |
J. He, P. Ni, C. Liu, Polym. Chem. 46 (2008) 3029-3041. DOI:10.1002/pola.22641 |
| [19] |
J. Jiang, H. Dong, Nat. Prod. Res. 22 (2008) 1410-1412. DOI:10.1080/14786410701823967 |
| [20] |
M. Bohorquez, C. Koch, T. Trygstad, N. Pandit, J.ColloidInterfaceSci. 216 (1999) 34-40. |
| [21] |
C. Xian, Z. Gu, G. Liu, J Wu, Chin. Chem. Lett (2019). DOI:10.1016/j.cclet.2019.09.011 |
| [22] |
S. Shetty, S. Udupa, L. Udupa, Evid. Complement. Altern. Med. 5 (2008) 95-101. DOI:10.1093/ecam/nem004 |
| [23] |
D. Wei, L.G. Hudson, K.J. Liu, Mol. Cell. Biochem. 279 (2005) 105-112. DOI:10.1007/s11010-005-8227-y |
 2020, Vol. 31
2020, Vol. 31 

