b Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China;
c College of Science, Department of Physics, Shanghai University, Shanghai 200444, China;
d College of Chemical Engineering, Hunan Chemical Vocational Technology College, Zhuzhou 310027, China
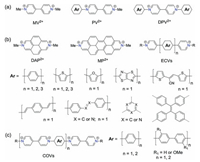
Viologens are a class of cationic organic compounds derived from 1, 1'-disubstituted-4, 4'-bipyridylium (BIPY) salts, which typically have rigid π-electron deficient structures and excellent reversible redox properties. In the early 1930s, Michaelis et al. initially reported the synthesis of 1'-disubstituted-4, 4'-bipyridylium (BIPY) salts. These compounds were named as "viologens" for their exhibited purple colors when treated with reducing reagents [1]. Over the past few decades, the π-electron-deficient feature and reversible redox properties have promoted viologens and their derivatives to be extensively applied in redox-tuned functional materials [2-4]. On the other hand, they also have been well recognized as fundamental supramolecular building blocks in wide aspects from macrocyclic hosts [5, 6], self-assembly systems [7-12], to molecular switches [13] and machines [14]. Compared with the tremendous application achievements of viologen, relatively less studies have been concerned on the structural modification of viologen compounds [15, 16]. In this content, a particularly interesting aspect is the extending of the π-conjugation of viologens. The resulted extended π-conjugated viologens (ECVs) [17-19] or π-conjugated oligomeric viologens (COVs) [20, 21] were found to exhibit interesting properties that are quite different from those of an isolated viologen unit. In general, there are three types of π-conjugated viologens as illustrated in Fig. 1. The simplest way to generate π-conjugated viologens is the introduction of aromatic substitutions (e.g., phenyl rings) to one or both sides of the N-termini (PV2+ and DPV2+ in Fig. 1a) [22-25]. However, the most attractive π-conjugated viologens are the socalled "extended viologens" whereby the two pyridinium residues in the molecular backbones are separated by aromatic rings (ECVs in Fig. 2b). So far, the polycyclic aromatic fused diazapyrene (DAP2+) or diazaperopyrenium (MP2+) dications [26, 27], as well as various ECV compounds have been synthesized and rapidly found widespread applications in supramolecular chemistry and materials science. In addition to the ECVs, efforts have also been devoted to the synthesis of COVs containing increasing numbers of alternately connected viologen and aromatic units (Fig. 1c). In the following sections, we will focus on the discussion of the recent development for ECV/COV compounds in the application of electrochromic and energy storage materials, supramolecular self-assembled systems, as well as covalent organic frameworks/ networks. It should be pointed out that the modification of extending the viologen units in the CBPQT4+ macrocycle has given birth to a novel class of fascinating ECV-based macrocycles [5, 28] or cages [29], which have already been summarized by Stoddart [5] recently and thus they are out of the scope of this review.
|
Download:
|
| Fig. 1. Typical chemical structures of π-conjugated viologens. (a) The N-termini aromatic (di-/phenyl) substituted viologens. (b) Extended π-conjugated viologens (ECVs) with pyridinium residues separated by aromatic rings. (c) π-conjugated oligomeric viologens (COVs) containing increasing numbers of viologen units connected by aromatic rings. | |
|
Download:
|
| Fig. 2. (a) Chemical structures of extended viologens 1-3. (b) Thiazolothiazole viologens fluorophores exhibiting strong fluorescence and viologen-like reversible electrochromism. Reproduced with the permission [18]. Copyright 2017, American Chemical Society. (c) Schematic representation of the [(NPr)2TTz]4+/NMe-TEMPO aqueous organic redox flow batteries (AORFB) and its anodic and cathodic half-cell reactions. Reproduced with permission [34]. Copyright 2018, Wiley-VCH. | |
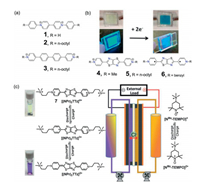
The pioneering attempts of extending π-conjugation of viologens were achieved by Vaid and coworkers. After the synthesis of phenyl viologen (1 in Fig. 2a) [30], they further reported the synthesis and characterization of an unexplored extended π-conjugated viologen compound (3 in Fig. 2a) by a straightforward procedure [17]. The electronic structure of ECV compound 3 was found to be closely related to that of the classic molecule Chichibabin's hydrocarbon, making compound 3 to be a stronger reducing agent than its isomer (2 in Fig. 2a). Moreover, they also synthesized a hexavalent conjugated viologen compound of hexakis(4-(N-butylpyridylium))benzene, which could serve as a six-electron organic redox system [31].
One significant application of viologen-based material is the rational design of molecular redox-flow batteries by taking advantages of the viologen compounds for the inexpensive and environmental benign organic electrolytes [32, 33]. It was found that the incorporation of aromatic heterocyclic units could generate new redox active ECV compounds, which are excellent candidates for the fabrication of new generation electrochromic or energy storage materials. For instance, Walter and co-workers have synthesized a series of thiazolothiazole (TTz) conjugated ECVs, which exhibited strong fluorescence and reversible electrochromism (Fig. 2b) [18]. The electronchemical properties of these unique ECVs were strongly affected by the fused bicyclic TTz heterocycle, through maintaining the planar conformation of the terminal pyridinium groups. The high quantum yields (0.8-0.96), combined with reversible electrochromic behavior, have made these TTzbased ECVs attractive materials with potential photochemical applications. Recently, Liu and co-workers have also prepared a TTz-based ECV compound [(NPr)2TTZ]Cl4 (7 in Fig. 2c), which could be used as a novel two-electron storage anolyte for aqueous organic redox flow battery (AORFB) (Fig. 2c) [34]. [(NPr)2TTZ]Cl4/NMe-TEMPO AORFB showed excellent battery performance, as reflected by both high energy efficiency and capacity retention per cycle. This work strongly speaks for the feasibility of designing new redox active compounds for AORFB applications by extending the π-conjugation of viologens.
In 2015, Baumgartner and co-workers have developed a simple Cu(II)-catalyzed procedure for the synthesis of N, N'-diarylated phosphaviologens (8-11 in Fig. 3a) whose extended π-systems possess an electron-deficient phosphole core [35]. Similar to the main features of typical viologen compounds, these unique phosphaviologens exhibited reversible chemical and/or electronic reduction to intensively colored and highly stable radical species. Specially, the thienyl-substituted compound 10 was found capable of absorbing light in the visible region due to the significantly diminished HOMO-LUMO gape. To show the potential application of these new compounds as electrochromic materials, the authors have further manufactured the multicolored proof-of-concept electrochromic devices, for which remarkable electrochromic properties could be observed intuitively (Fig. 3b). It is worthy of mentioning that this Cu(II)-catalyzed single-step synthetic method was compatible with both electron-poor and electron-rich diaryliodonium salts, which provides an accessible means to prepare π-extended viologens with electron-deficient N-aryl substitutions that are difficult to synthesize by classical Zincke reaction.

|
Download:
|
| Fig. 3. (a) The synthesis of π-extended electrochromic phosphaviologens. (b) The schematic representation for the reversible electrochromism of the proof-ofconcept device assembled from compound 10. Reproduced with permission [35]. Copyright 2015, American Chemical Society. | |
In addition to the ECVs, studies have been concerned on the synthesis of COVs containing polymeric 4, 4'-bipyridine units in the backbone. Recently, Colquhoun, Greenland and co-workers have demonstrated an efficient synthesis of conjugated 4, 4'-bipyridinium oligomers via the controlled stepwise Zincke reactions, from which a series of COV compounds (12-17 in Fig. 4a) containing increasing numbers of viologen units could be obtained [21, 36, 37]. Discrete and multiple redox processes as well as dramatic changes in electronic absorption spectra were observed for these oligomers. For instance, compound 17 exhibited four stable spectroscopically distinct species in the redox cycle (Fig. 4b); and compound 16 exhibited a noticeably narrower bandgap and showed photoconductivity under irradiation across a wide range of wavelengths in the visible spectral region. What is more, the methoxy-substituted COV congeners (12, 14 and 16) were found to be soluble in common organic solvents and could be processed by spin coating, this suggested these unique COV compounds may find use in optoelectronic devices.

|
Download:
|
| Fig. 4. (a) Chemical structures of rod-like COVs. (b) UV–vis spectral changes accompanying the stepwise reduction of compound 17 to a neutral species. Reprinted with permission [21]. Copyright 2016, The Royal Society of Chemistry. | |
By taking advantages of the combined electron-deficient feature and rigid conjugated structures, ECV molecules are excellent building blocks to be used in various supramolecular self-assembly systems with unique architectures or appealing application and functions. For example, aiming to explore more facial approaches in the fabrication of artificial folded and helical architectures, we have synthesized a pyridazine-bridged rigid rod-like tetracationic COV compound (18 in Fig. 5a), which could serve as an acceptor in templating the self-assembly of folded and helical supramolecular structures with a series of flexible linear naphthalene (NP) donor oligomers (Fig. 5a) [38]. We also prepared a series of novel fully rigid rod-rod type amphiphilic molecules (19 and 20 in Fig. 5b), in which the viologen unit playing the role of hydrophilic segment and the phenyl, biphenyl or para-tert-phenyl as a hydrophobic unit [39]. These novel ECV-based rigid amphiphiles could self-assembly into vesicles or microrods depending on their hydrophilic/ hydrophobic fraction ratios and the polarity of the solvents used (Fig. 5b). Moreover, it was found that the robustness of the selfassembled structures was enhanced, as a result of the rigid structures of the amphiphiles. These two examples have revealed the promising application of ECV/COV molecules in the construction of well-defined nanostructures, and it is expectable that novel functional self-assembled nanosystems could be obtained when the ECV/COV motifs are incorporated with specific functional groups.

|
Download:
|
| Fig. 5. (a) Donor-acceptor interaction-driven folding of linear NP oligomers templated by a rigid COV rod. Reproduced with permission [38]. Copyright 2015, the Royal Society of Chemistry. (b) Construction of vesicles, micro/nanorods and ultralong nanotubes through the self-assembly of ECV compounds as non-classical amphiphiles. Reproduced with permission [39]. Copyright 2017, Wiley-VCH. | |
In addition to small ECV compounds, the ECV-incorporated polymers have also proved to exhibit unprecedented self-assembly behaviors. Recently, Li and co-workers have synthesized a series of oligo(ethylene glycol)-linked ECV polymers (21–24 in Fig. 6a), in which the BIPY2+ units could be reduced into the radical cations (BIPY·+) by zinc powder [40]. The resulting ECV radical polymers were able to form pleated foldamers or a duplex via BIPY·+ dimerization. Moreover, when the NH4+ and Et3N were added alternately, reversible interconversion between the radical duplex and foldamer could be achieved. Such tunable pleated systems are of great significance for the potential applications in designing functional smart molecular materials. Furthermore, they also prepared rigid coplanar hydrazone-connected ECV polymers (25- 27 in Fig. 6b). After reducing the BIPY2+ units to BIPY·+ in acetonitrile, it was found that homoduplexes could be formed through the considerably face-to-face stacking of these coplanar radical ECV polymers. Specially, upon the addition of a square tetracationic cyclophane (28 in Fig. 6b) into the homoduplexes solution of 25, the stacked (BIPY·+)2 units could be encapsulated by the reduced radical cyclophane which stabilized the homoduplexes [41].

|
Download:
|
| Fig. 6. (a) Chemical structures and schematic diagram of the proposed pleated foldamers and a homoduplex of polymers 21–24 driven by the BIPY·+ dimerization and cationinduced conversion. Reproduced with permission [40]. Copyright 2015, The Royal Society of Chemistry. (b) Chemical structures of the rigid coplanar ECV polymers 25-27, and the schematic representation of their radical stacking into homoduplexes, as well as the encapsulation enhanced radical stacking of 25 with square bis(radical cation) cyclophane 28. Reproduced with permission [41]. Copyright 2016, Taylor & Francis Group. | |
One of the most attractive features for the viologen derivatives is their host-guest encapsulation behaviors with macrocyclic molecules in aqueous phase. In this context, the cucurbit[n]urils (specially CB[8]) are among the most extensively explored macrocycles in the construction of numerous viologen/CB[n] host-guest systems. For example, the water-soluble ECV molecules were found to exhibit specially dynamic host-guest complexation behaviors with CB[8] in water [24, 42], unique fluorescent[2]rotaxane [25] and pseudorotaxanes [43], or triangular[4]molecular necklaces [44] could also be constructed from the ECV molecules. Recently, Zhang and co-workers have demonstrated that two radical cation of 4·+ could be encapsulated by CB[8] to generate a supramolecular radical dimer featuring with highefficiency NIR-II photothermal conversion and improved stability, which enabled such supramolecular radical dimer to be used in photothermal therapy [45, 46].
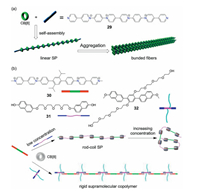
However, the most distinctive applications of the ECV molecules with CB[n] hosts in water are the fabrication of CB[8]-mediated host-guest supramolecular polymers [12, 47]. In 2014, we initially reported the construction of linear supramolecular polymers with rigid backbones via the CB[8]-encapsulation enhanced head-to-tail stacking of the ECV monomer (29 in Fig.7a) [48]. The resulting linear supramolecular polymer was found capable of aggregating into stick-like bunched fibers which could be clearly visualized by SEM and TEM. The use of such rigid ECV monomers has provided a promising strategy in fabricating supramolecular polymers featured with the space distance of the substituents being accurately controlled, which is difficult achieved for the vast majority of flexible supramolecular monomers.
|
Download:
|
| Fig. 7. (a) Chemical structure of rigid tetracationic ECV monomer 29, and cartoon representation of the construction of rigid supramolecular polymer (SP) in water through the self-assembly of rod-like monomers and CB[8]. Reproduced with permission [48]. Copyright 2014, The Royal Society of Chemistry. (b) Cartoon representation of formation of the supramolecular bottlebrush polymer coassembled from COV monomer 30, electron-rich monomers 31 or 32 and CB[8], respectively. Reproduced with permission [49] and [50]. Copyright 2017, Chinese Academy of Science; Copyright 2017, Elsevier. | |
For the purpose to extend the structural diversity of water soluble supramolecular polymers, we further prepared the tetracationic COV monomer (30) which was used to co-assembly with an electron-rich flexible monomer (31) to form a rod-coil supramolecular copolymer driven by CB[8]-encapsulation-enhanced donor-acceptor interaction (Fig. 7b) [49]. The resulting supramolecular copolymer exhibited interesting concentration dependent morphology, as changing from extended linear conformation at low concentration to the curled aggregated morphology at high concentration. Moreover, when the COV monomer 30 was co-assembled with the rigid electron-rich monomer 32 in the presence of CB[8] in water, a unique rigid supramolecular bottle brush polymer could be constructed (Fig. 7b) [50]. These two examples provide facile approaches in the fabrication of water-soluble supramolecular copolymers featured with unique architectures, which may find potential applications in the field of biomaterials.
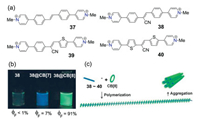
The radical cations of viologens were able to undergo dimerization or pimerization through the stacking interactions between the BIPY·+ units, which has been emerged as a new noncovalent interaction in the construction of supramolecular architectures in the last decade [10]. Aiming to investigate the unexplored properties and applications of the conjugated viologens, we have synthesized a series of novel COV compounds whose viologen units were connected by phenyl (33, 34) or biphenyl (35, 36) linkers (Fig. 8) [20]. The electrochemical and photophysical properties of such COVs were found to be distinguished from those of isolated viologen unit. More interestingly, when these compounds were reduced to their radical cations by sodium dithionite, linear supramolecular radical polymers could be formed through the strong radical dimerization of the monomers even in quite dilute conditions (5×10-4 mol/L) (Fig. 8). It was also found that the addition of 1 equiv. CB[8] was able to promote the radical dimerization to form host-enhanced supramolecular radical polymers. This work not only well illustrated the structure-properties relationships of the COV compounds, but also indicated the potential application of COV radical compounds in the construction of novel redox-switchable assembly systems. Another fascinating application of the ECV/COV-CB[8] selfassembly systems is the design of fluorescent supramolecular polymers with tunable light-emitting properties. In 2016, Park and co-workers have designed and synthesized a series of cyanostilbene-derived ECV compounds (37-40 in Fig. 9a), which could be used to construct both supramolecular polymers with highly enhanced fluorescence intensity [51], and novel light-harvesting supramolecular block copolymers (SBCPs) in water [52]. As showed in Fig. 9b, monomer 38 was found to exhibit weakfluorescent (ΦF < 1%) in aqueous solution, whereas moderate blue monomeric emission (ΦF = 7%) could be observed for the 1:2 inclusion complexes of 38@CB[7]. However, bright fluorescence (ΦF = 91%) was observed when equivalent of CB[8] was introduced to the solution of 38. The highly fluorescent phenomenon could be attributed to the formation of linear rigid supramolecular polymers (SPs) which enabled the strong J-aggregation between the cyanostilbene chromophores of the monomers (Fig. 9c). This could be further evidenced by the observed supramolecular polymerization inhibition and fluorescent quench for the hostguest complex of non-cyano-unit derived monomer 37 and CB[8]. Based on these findings, they have demonstrated that supramolecular homopolymers (SHPs) with finely tunable colors could be obtained when different cyanostilbene guests (38-40 in Fig. 9a) were used [52]. Moreover, highly efficient light-harvesting supramolecular block copolymers (SBCPs) were further fabricated by mixing the blue (as donor) and red-emitting (as acceptor) SHPs. This cyanostilbene-based ECV/CB[8] system shows great potential in wide application from ecofriendly light-driven fuel productions to photodynamic therapy.

|
Download:
|
| Fig. 8. Chemical structures of linear COVs 33-36 and the cartoon representation of the formation of rigid linear supramolecular radical polymers and their further aggregation into bundles (COV compounds 33 and 35 were used as the representatives). Reproduced with permission [20]. Copyright 2016, the Royal Society of Chemistry. | |
|
Download:
|
| Fig. 9. (a) Chemical structures of diarylethene-bridged ECV monomers 37-40. (b) Fluorescence images and ΦF values of 38, 38@CB[7] and 38@CB[8] complexes in water. (c) Schematic representation of the formation of CB[8]-mediated host-guest supramolecular polymer. Reproduced with permission [51]. Copyright 2016, WileyVCH. | |
In addition, the ECV/CB[8] system could be applied in the construction of more challenging multicolor photoluminescence materials with external stimuli controlled luminescence color conversion behaviors. In 2016, Ni and coworkers have synthesized a water-soluble ECV molecule (41) incorporated with an oligo(p-phenylenevinylene) (OPV) core (Fig. 10a) [53]. This OPV-bridged chromophore guest could be encapsulated by the hydrophobic cavity of CB[8], and linear supramolecular polymer could be formed upon the addition of increasing concentrations of CB[8] host, during which the solution exhibited tunable fluorescent emissions including cyan, yellow, green, and white (Fig. 10b). Moreover, it was found the emission behavior of such system was pH-dependent. Tunable luminescence was observed at a certain pH range (4.6–5.5) due to two coexisting host-guest interactions between CB[8] and 41 (Fig. 10a). The establishment of such stimulus-responsive color tunable ECV/CB[8] self-assembly system has provided a simple supramolecular toolbox for producing light-emitting materials.
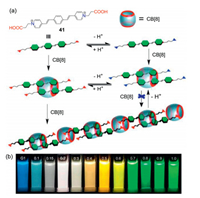
|
Download:
|
| Fig. 10. (a) Chemical structure of the OPV-bridged water soluble ECV compound 41, and the schematic representation of the CB[8]-based approach for fabricating smart luminescent materials, showing host-guest interactions of CB[8] with 41 that lead to different supramolecular assemblies in water. (b) Photographs of solution of 41 (10.0 μmol/L) upon addition of increasing concentrations of CB[8] host (0–1.0 equiv.) in aqueous media (pH 2.0) under UV light at 365 nm. Reproduced with permission [53]. Copyright 2016, American Chemical Society. | |
During the past decade, a major thrust in materials science is the design and synthesis of 2D organic materials, which exhibit unique graphene-like topological features and attractive unprecedented properties. Researches in the synthetic 2D organic materials have now boomed as an important branch of modern materials chemistry [54-56]. In this context, one of the hot topics is the development of 2D porous framework materials represented by 2D metal-organic frameworks (MOFs) [57, 58], 2D covalent organic frameworks (COFs) [59], as well as 2D supramolecular organic frameworks (SOFs) [60-62]. Although both 2D MOFs and COFs have shown great potential applications in widespread aspects, their porous structures and properties are always displayed in the solid state, and the insolubility has greatly limited them from processing use as the traditional polymers did.
To address this problem, self-assembly provides a straightforward and efficient strategy for the construction of soluble periodic porous supramolecular organic frameworks (SOFs) in solution (typically in water) from rationally designed molecular building blocks. Notably, the pyridinium derivatives with rigid conjugation structures are the most extensively used building blocks which have played a central role in the fabrication of various 2D/3D SOFs. In 2013, Li, Liu, Zhao and co-workers have demonstrated the first successful construction of a water soluble 2D honeycomb SOF through the co-assembly of a C3-symmmetric rigid tricationic pyridinium derivative and CB[8] [63]. Inspired by this pioneering work, a variety of symmetric tri-and tetra-armed COV compounds were synthesized and successfully applied in the construction of novel water soluble well-defined 2D SOFs with different topologies, many of which have exhibited attractive unprecedented properties and functions. For instance, Li and co-workers have further synthesized a hexacationic COV monomer (42 in Fig. 11a) whose BIPY2+ units could be reduced to the BIPY·+ units, from which 2D SOF driven by the radical-dimerization of (BIPY·+)2 units were generated. The addition of CB[8]s were found to further stabilized the resulting radical SOF via encapsulating the (BIPY·+)2 units (Fig. 11b) [64]. Particularly, the highly soluble 42 could also be used to construct CB[8]-encapsulation-enhanced 2D single layered SOFs through the co-assembly with linear flexible electron-rich monomers 45 and 46 in water, respectively [65]. It was worthy of mentioning that these ternary 2D SOFs exhibited antimicrobial activity, and the one constructed from 42 and 46 displayed unique pH-regulated dis-/assembly behaviors benefiting from the pHresponsiveness of the amine groups of 46. In addition to the hexagonal pores, we discovered that the four-armed square shaped porphyrin-based COV monomer (43 in Fig. 11a) was able to coassembly with the flexible monomers of 44 and 45, respectively, to form distinctive 2D SOFs with parallelogram pore architectures (Fig. 11c) [66]. These SOFs exhibited interesting softness because of the use of flexible building blocks.
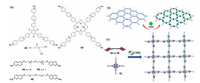
|
Download:
|
| Fig. 11. (a) Chemical structures of the hexacationic tri-armed COV monomer 42 and the octcationic four-armed COV monomer 43, as well as the linear flexible monomers 44- 46 used in the construction of 2D SOFs. (b) Schematic presentation of the honeycomb-styled 2D SOF formed by the stacking 42 tri-radicals and further promoted by CB[8]. Reproduced with permission [64]. Copyright 2014, the Royal Society of Chemistry. (c) Schematic representation of a 2D supramolecularly polymeric pattern from the selfassembly of 43 with 42 or 45 in the presence of CB[8]. Reproduced with permission [66]. Copyright 2014, the Royal Society of Chemistry. | |
During the past few years, another significant achievement in this field is the fabrication of fluorescent 2D SOFs through the selfassembly of rationally designed ECV monomers. In this context, tetraphenylethene (TPE)-based ECV compounds are the most frequently used monomers, since the TPE scaffold is wellrecognized as one of the most popular AIEgens [67]. In 2015, we reported that a TPE-based tetracationic pyridinium-derived monomer (non-emissive) was able to assembled with CB[8] to form an emissive 2D SOF [68], whose fluorescence turn-on behavior could be owned to the self-assembly induced free rotation restriction of the TPE units. Meanwhile, Liu and coworkers have also discovered that the self-assembly of this TPEbased tetracationic pyridinium monomer and CB[8] could form supramolecular hydrogel with bright orange fluorescent emission and highly thermostability [69]. Very recently, by using another TPE-based ECV molecule equipped with four pyridiniumvinyl arms (47 in Fig. 12a), Zhao and co-workers have demonstrated the an AIE-featured 2D SOF. High affinity for DNA and remarkably enhanced fluorescence could be observed for such TPE-type ECV molecule, which makes it an excellent biological fluorophore staining of intracellular DNA imaging of living cells (Fig. 12b) [70].
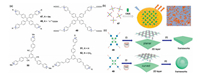
|
Download:
|
| Fig. 12. (a) Chemical structures of the representative reported ECV monomers used in the construction of fluorescent 2D SOFs. (b) Formation of fluorescent two-dimensional supramolecular structure based on TPE derivative 47 and CB[8]. Reproduced with permission [70]. Copyright 2019, the Royal Society of Chemistry. (c) Cartoon representation of the hierarchical formation of supramolecular network-type layers and frameworks from the self-assembly of 48 or 49 with CB[8]: (i) host-guest complexation; (ii) stacking or aggregating. Reproduced with permission [71]. Copyright 2018, Wiley-VCH. | |
Cao and co-workers have reported another two TPE-based ECV molecules with four pyridinium (48 in Fig. 12a) or pyridiniumvinyl (49 in Fig. 12a) arms, both of which could be used in the construction of 2D SOFs [71]. As displayed in Fig. 12c, while 48 and CB[8] self-assembled into planar SOF layer and further stacked into cuboid frameworks, the self-assembly of 49 and CB[8] led to the formation of curved SOF layer and further aggregated into spheroid frameworks. This unique shape-controllable self-assembly behavior could be owned to the slight difference on the pyridinium (vinyl) arms of the monomers. It was found these SOFs were fluorescent with large red-shifts (up to 82 nm). Moreover, competitive guest stimuli-responsive off/on fluorescence could be achieved, the turn-on of which was further employed in cellular imaging.
In spite of TPE-type ECV molecules, several tritopic ECV molecules were also prepared for constructing fluorescent 2D SOFs. For example, Park and co-workers have also demonstrated the construction of a highly fluorescent 2D SOF by employing a trilateral cyanstilbene derived ECV compound as monomer (50 in Fig. 12a) [72]. The obtained 2D SOF was further used as a photosensitizer template in photocatalytic H2 evolution by improving the turnover number of a colloidal Pt-catalyst. Very recently, Li, Zhou, Zhao and co-workers have synthesized a triarmed ECV molecule containing a benzene core and three Brooker's merocyanine (BM) analogs as arms (51 and 52 in Fig. 12a) [73]. Water soluble 2D SOF could be obtained through the strong host-guest interactions between the BM arms of 52 and CB[8], which exhibited obvious AIE enhancement effect in H2O. It should be pointed out that ECV compound 52 was observed to exhibit novel DNA induced photoluminescence enhancement, making the 52-CB[8] fluorescent 2D SOF to be a promising fluorescent probe for intracellular DNA imaging.
On the basis of the similar designing principle, threedimensional supramolecular organic frameworks (3D SOFs) with diamondoid and cubic geometries could also be generated through the co-assembly of CB[8] and tetrahedral monomers. In this context, the Li group has devoted great efforts in the structural design and application exploration of 3D SOFs. By employing the tetrahedral monomers with four monocationic pyridinium units, they initially designed and prepared a series of homogeneous 3D SOFs as well as the distinctive metal-coordination hybrid 3D MSOFs. Benefiting from the large internal cage-like cavities and the positive charged building blocks, these 3D SOFs and MSOFs have revealed wide applications from anionic guest adsorption and drug delivery [74-77], to 3D light-harvesting systems using in homogeneous and heterogeneous photocatalysis [78-82]. Recently, Li and co-workers demonstrated that when the ECV motifs were incorporated into the tetrahedral monomers, the pore-expanded 3D SOFs could be fabricated. For instance, by using the ECVmodified tetrahedral monomer 53 (Fig. 13), they have constructed a 3D SOF with a 3.6 nm-aperture in water [83]. The large pore sizes not only facilitated the adsorption of Ru2+ complex photosensitizers and polyoxometalates (POMs), but also enhanced the photocatalytic activities compared to those prototypical 3D SOFs with smaller aperture.

|
Download:
|
| Fig. 13. Chemical structures of the tetrahedral ECV compound 53, (b) Schematic illustration of the assembly of 53 and CB[8] into 3D SOF-bpb in water and the subsequent coadsorption of Ru-photosensitizer (red) and polyoxometallate catalyst (blue) to form (Ru2+ & WD-POM)⊂SOF. Reproduced with the permission [83]. Copyright 2019, the Royal Society of Chemistry. | |
From these examples we can conclude that the ECV-type monomers have played a crucial role in the fabrication of 2D/3D SOFs. Although the SOFs library is relatively small in comparison to the MOFs and COFs congeners, this special kind of supramolecular material has already drawn increasing attention and begun to show novel properties and functions. We believe researches on SOFs will undoubtably spring up, and the ECV molecules will also keep playing the irreplaceable role in both structural and functional exploration of new SOFs.
3. ECV-Based covalent organic networksThe past decade has witnessed the rapid development of organic porous materials with extended covalent organic networks in the form of either amorphous covalent organic polymers (COPs) [84, 85] or crystalline covalent organic frameworks (COFs) [59, 86, 87], for which widespread applications have been extensively explored from gas adsorption and separation, drug delivery, catalysis, energy storage and so on. However, the vast majority of the reported organic porous materials are prepared from neutral building blocks, only few examples are synthesized with ionic monomeric molecules [88-94]. Although continued attention has been paid on the viologen-based polymeric materials in which the positively charged and reversible redox behavior of the viologen units may endow them with unique properties and function [4, 95-97]. Viologen-based polymeric materials with ordered network structures are relatively rare for the lack of efficient synthetic methods. Nevertheless, several examples reported recently have showed that the incorporated charged building blocks have endowed the viologen-based covalent organic networked polymers with unique tunable properties and functions.
In 2016, Li and co-workers have demonstrated that a polycationic 2D COF with a pore diameter of about 5.8 nm could be synthesized through the condensation reaction of aromatic triangular triamine monomer 54 and a linear viologen-derived dialdehyde monomer 55, (Fig. 14) [98]. The honeycomb PC-COF layers were found to stack into 3D porous frameworks with the eclipsed patterned adjacent BIPY2+ units, and the chloride counterions were found being sandwiched between the layers. The positive-charged feature has enabled the PC-COF to be served as a robust adsorbent capable of efficiently (capacity > 97%) uptaking various anionic organic dye pollutants at a low concentration (3.2×10-5 mol/L).

|
Download:
|
| Fig. 14. The synthesis of 2D honeycomb-styled PC-COF from the condensation of triamine monomer 54 and ECV dialdehyde 55. Reproduced with the permission [98]. Copyright 2016, The Royal Society of Chemistry. | |
Although the application of Zincke reaction has been limited in the synthesis of small viologen molecules or conjugated oligomeric viologens, recently Trabolsi and co-workers have showed that this reaction could also be used for the preparation of viologen-linked covalent organic networks under solvothermal or microwave conditions [99]. As described in Fig. 15, when the rigid triamine 54 was reacted with dicatinic Zincke salt 56 in a binary solvent of ethanol/water (4/1, v/v) or in pure dioxane either solvothermal or microwave heating, hollow spherical (HS) particles or hollow tubelike (HT) structures were obtained, respectively. However, a crystalline covalent organic gel framework (COGF) could be formed when the reaction was performed in ethanol/water (1/1, v/v) under microwave irradiation. It was found that these covalent organic networked viologen materials exhibited not only good resistant to moisture but also high stability in acidic or basic aqueous solutions. Moreover, the authors also showed that viologen-based covalent organic nanosheets (CONs) and covalent organic tubes (COTs) could be prepared via template-free Zincke reaction of the same viologen linker with different pyrene-based cores, respectively, which facilitated their application as efficient adsorbents for iodine both in solution and vapor phase [100]. It was acceptable that the morphologies of the covalent organic networks have played a pivotal role in determining their functions and potential applications. These works have provided rational strategies in the challengeable adjustment of the morphology of covalent organic nanomaterials.

|
Download:
|
| Fig. 15. Synthesis of the viologen-based self-templated materials via Zincke reaction under solvothermal conditions (ST) or microwave (MW) irradiation: (a) amorphous hollow spheres (HS) and tubes (HT) and (b) a crystalline covalent organic gel framework (COGF). Reproduced with permission [99]. Copyright 2017, American Chemical Society. | |
In addition to the positively charged feature, the excellent redox properties of viologen units have also provide covalent organic networked polymers with unprecedented functions. Recently, Wen, Yang and co-workers have synthesized a 2D redox-active cationic covalent triazine network (cCTN) by employing the Zincke reactions between the dicationic salt 56 and a rigid benzonitrilecored triamine 57 (Fig. 16a) [101]. The obtained viologen-based cCTN could be used directly as a metal-free electrocatalyst for the oxygen reduction reaction (ORR) to selectively produce H2O2 from O2. The EPR experiments revealed that the viologen units in the cCTN could be reduced to radical cations accompanied by the generation of super oxygen radicals, which enabled an efficient two-electron pathway ORR process in the polymers. It was worthy of mentioning that this viologen-based cCTN materials provided a more green and safe strategy for the direct electrosynthesis of hydrogen peroxide compared to the traditional anthraquinone process. Besides, Ghosh and co-workers further discovered that this viologen-based cCTN was able to be used as an efficient scavenger of toxic oxo-anions (CrO42-, MnO4-, ReO4-) from water (Fig. 16b) [102].

|
Download:
|
| Fig. 16. (a) Synthesis of a redox-active cationic covalent triazine network (cCTN) via a Zincke reaction for the selective electrosynthesis of H2O2 from O2. Reproduced with permission [101]. Copyright 2018, the Royal Society of Chemistry. (b) Schematic representation of the oxo-anion capture in ionic viologen-organic network. Reproduced with permission [102]. Copyright 2018, the Royal Society of Chemistry. (c) Synthetic route for the preparation of charged covalent triazine frameworks (cCTFs). Reproduced with permission [103]. Copyright 2017, American Chemical Society. | |
One of the most attractive applications of conjugated organic porous polymers is their ability of capturing CO2 and even converted it into value-added products. In this aspect, viologenbased covalent organic networked materials have also revealed their potential values in the development of sustainable and environmental-friendly materials for CO2 capture and fixation. For instance, Coskun and co-workers have demonstrated that the ZnCl2 catalyzed trimerization reactions of the nitrile functionalized dicationic viologen monomer 58 could lead to the formation of charged covalent triazine frameworks (cCTFs) (Fig. 16c) [103]. This viologen-based cCTFs exhibited both high specific surface areas (up to 1247 m2/g) and high physicochemical stability. More importantly, the presence of positively charged viologen units in the cCTFs have substantially promoted their CO2 affinity (up to 133 mg/g at 1 bar and 273 K), making them being hierarchically porous organocatalysts for CO2 conversion. They also prepared a series of viologen-incorporated porous cationic polymers (PCPs) with different counter anions through the efficient palladium catalyzed cross coupling reaction between 59 and 60 (Fig. 17a) [104]. These viologen-based PCPs were found to be excellent porous organocatalysts for conserving CO2 into cyclic carbonates. Moreover, both the gas sorption properties and catalytic activity of these porous polymers could be regulated by simply varying the counter anions within the PCPs networks.

|
Download:
|
| Fig. 17. (a) The synthetic strategy for the preparation of viologen-incorporated porous cationic polymers (PCPs) with different counter anions and their applications of CO2 capture and conversion. Reproduced with permission [104]. Copyright 2016, the Royal Society of Chemistry. (b) The synthesis of POSS and viologen-linked porous cationic frameworks by the Zincke reaction towards catalytic conversion of CO2. Reproduced with permission [105]. Copyright 2018, The Royal Society of Chemistry. | |
Recently, Chen, Long and co-workers have reported the preparation of novel viologen-based organic-inorganic hybrid porous cationic frameworks by using the polyhedral oligomeric silsesquioxanes (POSS) (61 in Fig. 17b) as viologen inter-linkers [105]. The existence of the POSS cages have equipped such viologen-POSS-based porous polymers with high surface areas, abundant ionic sites as well as enriched Si-OH groups, which facilitated the simultaneous CO2 capture and catalytic cycloaddition of CO2 with epoxides under mild conditions.
Non-conjugated viologen-based covalent organic frameworks or networks could also be fabricated from the ECV monomers and selected linkers. For example, Trabolsi and co-workers have used the Zincke salt 56 and the hexatopic phosphazene cored amines to prepare flexible viologen-based covalent organic polymers, which exhibited multifunctional redox-tuned applications in magic printing, gaseous ammonia sensing and oxoanion capture [106]. They also found that redox-responsive covalent organic polymeric nanosheets could be synthesized from linear ECV-type diamines and nitro-derived calix[4]arene capture [107]. The backbone rigidity and redox states of the prepared viologen-based COPs could be tuned through the regulation of the redox states of the viologen units, and the resulting polymers exhibited different uptake capacities for pollutants of iodine and toxic dye in water.
4. Summary and outlooksIn this review, we have briefly summarized the recent significant progresses of extended π-conjugated viologen derivatives and their applications as supra-/molecular building blocks in the fields of electrochromic and energy storage materials, supramolecular self-assembly and organic porous polymers. Among them, distinctive structural features and unique physical-chemical properties have made the ECV molecules to be promising candidates in the construction of functional supramolecular polymers (SPs). In particular, they have been proved to be one of the most important building blocks for 2D/3D supramolecular organic frameworks in aqueous solution. Moreover, covalent organic frameworks or networks incorporated with π-conjugated viologens have also been successfully fabricated which exhibited attractive properties and functions including efficient pollutants adsorption and separation in water, metal-free electron catalysis, as well as high-performance of CO2 adsorption and conversion. All these achievements have indicated bright future for this new class of molecules with advantages, potentials and expectations.
Nevertheless, certain obstacles are undoubtedly existing that need to be addressed. Meanwhile, the application scopes of these molecules are still limited, and more broad areas with exciting applications are worthy of exploration. First of all, despite of the currently relied Zincke type reactions, more efficient synthetic methods are highly demanded for the structural diversities of ECV/ COV molecules. Secondly, more appropriate stimuli sources are encouraged to introduce to the self-assembled ECV/COV-based SPs or SOFs systems, so as to construct smart supramolecular materials with fascinating functions. Last but not least, for the viologenbased polymeric materials, it is well worth to shift the amorphous viologen-based covalent organic polymers toward crystalline COFlike materials, for it may give birth to more application opportunities. Recent attempt has provided another suggestion that fascinating unexplored properties and functions could be obtained by incorporating the host-guest complexation of ECVhost pair within the ordered frameworks or networks [108]. In conclusion, we do hope the present summarized manuscript will not only provide a groundwork for the rational design and construction of new generation supra-/molecular building blocks, but also continue to promote much more researches in the future.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsT.G. Zhan and K.D. Zhang are acknowledged for the financial supports of the Natural Science Foundation of Zhejiang Province (No. LY20B020005) to this research. X. Zhao thanks Shanghai Scientific and Technological Innovation Project (No. 18JC1410600) for the financial support.
| [1] |
L. Michaelis, E.S. Hill, J. Gen. Physiol. 16 (1933) 859-865. DOI:10.1085/jgp.16.6.859 |
| [2] |
L. Striepe, T. Baumgartner, Chem. -Eur. J. 23 (2017) 16924-16940. DOI:10.1002/chem.201703348 |
| [3] |
K. Madasamy, D. Velayutham, V. Suryanarayanan, M. Kathiresan, K.C. Ho, J. Mater. Chem. C 7 (2019) 4622-4637. DOI:10.1039/C9TC00416E |
| [4] |
T. Škorjanc, D. Shetty, M.A. Olson, A. Trabolsi, ACS Appl. Mater. Interfaces 11 (2019) 6705-6716. DOI:10.1021/acsami.8b20743 |
| [5] |
E.J. Dale, N.A. Vermeulen, M. Jurícek, et al., Acc. Chem. Res. 49 (2016) 2629-273. |
| [6] |
Z. Liu, S.K.M. Nalluri, J.F. Stoddart, Chem. Soc. Rev. 46 (2017) 2459-2478. DOI:10.1039/C7CS00185A |
| [7] |
B. Zheng, F. Wang, S. Dong, F. Huang, Chem. Soc. Rev. 41 (2012) 1621-1636. DOI:10.1039/C1CS15220C |
| [8] |
D. Qian, F. Yan, Progress in Chemistry 25 (2013) 46-53. |
| [9] |
Y. Liu, H. Yang, Z. Wang, X. Zhang, Chem. -Asian J. 8 (2013) 1626-1632. DOI:10.1002/asia.201300151 |
| [10] |
D.W. Zhang, J. Tian, L. Chen, L. Zhang, Z.T. Li, Chem. -Asian J. 10 (2015) 56-68. DOI:10.1002/asia.201402805 |
| [11] |
Y. Chen, F. Huang, Z.T. Li, Y. Liu, Sci. China Chem. 8 (2018) 979-998. |
| [12] |
H.D. Correia, S. Chowdhury, A.P. Ramos, et al., Polym. Int. 68 (2019) 572-588. DOI:10.1002/pi.5709 |
| [13] |
Z.J. Zhang, H.Y. Zhang, L. Chen, Y. Liu, J. Org. Chem. 76 (2011) 8270-8276. DOI:10.1021/jo201441r |
| [14] |
Y. Wang, M. Frasconi, J.F. Stoddart, ACS Cent. Sci. 3 (2017) 927-935. DOI:10.1021/acscentsci.7b00219 |
| [15] |
M. Stolar, J. Borau-Garcia, M. Toonen, T. Baumgartner, J. Am. Chem. Soc. 137 (2015) 3366-3371. DOI:10.1021/ja513258j |
| [16] |
C. Reus, M. Stolar, J. Vanderkley, J. Nebauer, T. Baumgartner, J. Am. Chem. Soc. 137 (2015) 11710-11717. DOI:10.1021/jacs.5b06413 |
| [17] |
W.W. Porter, T.P. Vaid, A.L. Rheingold, J. Am. Chem. Soc. 127 (2005) 16559-16566. DOI:10.1021/ja053084q |
| [18] |
A.N. Woodward, J.M. Kolesar, S.R. Hall, et al., J. Am. Chem. Soc. 139 (2017) 8467-8473. DOI:10.1021/jacs.7b01005 |
| [19] |
J. Luo, B. Hu, C. Debruler, T.L. Liu, Angew. Chem. Int. Ed. 57 (2018) 231-235. DOI:10.1002/anie.201710517 |
| [20] |
T.G. Zhan, T.Y. Zhou, F. Lin, et al., Org. Chem. Front. 3 (2016) 1635-1645. DOI:10.1039/C6QO00298F |
| [21] |
L. Chen, H. Willcock, C.J. Wedge, et al., Org. Biomol. Chem. 14 (2016) 980-988. DOI:10.1039/C5OB02211H |
| [22] |
A. Iordache, M. Oltean, A. Milet, et al., J. Am. Chem. Soc. 134 (2012) 2653-2671. DOI:10.1021/ja209766e |
| [23] |
F. Benyettou, X. Zheng, E. Elacqua, et al., Langmuir 32 (2016) 7144-7150. DOI:10.1021/acs.langmuir.6b01433 |
| [24] |
G. Wu, M. Olesinska, Y. Wu, D. Matak-Vinkovic, O.A. Scherman, J. Am. Chem. Soc. 139 (2017) 3202-3208. DOI:10.1021/jacs.6b13074 |
| [25] |
Y. Yu, Y. Li, X. Wang, et al., J. Org. Chem. 82 (2017) 5590-5596. DOI:10.1021/acs.joc.7b00400 |
| [26] |
A.N. Basuray, H.P.J. de Rouville, K.J. Hartlieb, et al., Angew. Chem. Int. Ed. 51 (2012) 11872-11877. DOI:10.1002/anie.201205089 |
| [27] |
A.N. Basuray, H.P.J. de Rouville, K.J. Hartlieb, A.C. Fahrenbach, J.F. Stoddart, Chem. -Asian J. 8 (2013) 524-532. DOI:10.1002/asia.201200780 |
| [28] |
J.C. Barnes, M. Jurícek, N.A. Vermeulen, E.J. Dale, J.F. Stoddart, J. Org. Chem. 789 (2013) 11962-11969. |
| [29] |
E.J. Dale, N.A. Vermeulen, A.A. Thomas, et al., J. Am. Chem. Soc. 136 (2014) 10669-10682. DOI:10.1021/ja5041557 |
| [30] |
W.W. Porter, T.P. Vaid, J. Org. Chem. 70 (2005) 5028-5035. DOI:10.1021/jo050328g |
| [31] |
Z. Han, T.P. Vaid, A.L. Rheingold, J. Org. Chem. 73 (2008) 445-450. DOI:10.1021/jo701944c |
| [32] |
S. Sathyamoorthi, M. Kanagaraj, M. Kathiresan, V. Suryanarayanana, D. Velayutham, J. Mater. Chem. A 4 (2016) 4562-4569. DOI:10.1039/C6TA00858E |
| [33] |
B. Hu, C. DeBruler, Z. Rhodes, T.L. Liu, J. Am. Chem. Soc. 139 (2017) 1207-1214. DOI:10.1021/jacs.6b10984 |
| [34] |
J. Luo, B. Hu, C. Debruler, T.L. Liu, Angew. Chem. Int. Ed. 57 (2018) 231-235. DOI:10.1002/anie.201710517 |
| [35] |
C. Reus, M. Stolar, J. Vanderkley, J. Nebauer, T. Baumgartner, J. Am. Chem. Soc. 137 (2015) 11710-11717. DOI:10.1021/jacs.5b06413 |
| [36] |
L. Chen, F. Hartl, H.M. Colquhoun, B.W. Greenland, Tetrahedron Lett. 58 (2017) 1859-1862. DOI:10.1016/j.tetlet.2017.03.089 |
| [37] |
L. Chen, E. Vivier, C.J. Eling, et al., Synth. Met. 241 (2018) 31-38. DOI:10.1016/j.synthmet.2018.03.019 |
| [38] |
T.G. Zhan, B.Y. Lu, F. Lin, et al., Org. Chem. Front. 2 (2015) 1578-1583. DOI:10.1039/C5QO00244C |
| [39] |
F. Lin, R.R. Liang, Q.Y. Qi, et al., Chin. J. Chem. 35 (2017) 429-434. DOI:10.1002/cjoc.201600906 |
| [40] |
Y.C. Zhang, D.W. Zhang, H. Wang, Y. Zhou, Z.T. Li, Polym.Chem. 6 (2015) 4404-4408. DOI:10.1039/C5PY00419E |
| [41] |
Q. Qi, C.G. Xi, H. Wang, D.W. Zhang, Z.T. Li, Supramol. Chem. 28 (2016) 762-767. DOI:10.1080/10610278.2016.1165348 |
| [42] |
K.J. Hartlieb, A.N. Basuray, C. Ke, et al., Asian J. Org. Chem. 2 (2013) 225-229. DOI:10.1002/ajoc.201200187 |
| [43] |
Y. Song, X. Huang, H. Hua, Q. Wang, Dyes Pigm. 137 (2017) 229-235. DOI:10.1016/j.dyepig.2016.10.012 |
| [44] |
S.K. Samanta, K.G. Brady, L. Isaacs, Chem. Commun. (Camb.) 53 (2017) 2756-2759. DOI:10.1039/C6CC10328F |
| [45] |
Y. Yao, R. Zhao, Y. Shi, et al., Chem. Commun. (Camb.) 54 (2018) 8068-8071. DOI:10.1039/C8CC04423F |
| [46] |
B. Tang, W.L. Li, Y. Chang, et al., Angew. Chem. Int. Ed. 58 (2019) 15526-15531. DOI:10.1002/anie.201910257 |
| [47] |
W. Huang, T.G. Zhan, F. Lin, X. Zhao, Prog. Chem. 28 (2016) 165-183. |
| [48] |
F. Lin, T.G. Zhan, T.Y. Zhou, et al., Chem. Commun. (Camb.) 50 (2014) 7982-7985. DOI:10.1039/C4CC02971B |
| [49] |
Y. Fan, F. Lin, X.N. Xu, J.Q. Xu, X. Zhao, Acta Polym. Sin. (2017) 80-85. http://en.cnki.com.cn/Article_en/CJFDTOTAL-GFXB201701010.htm
|
| [50] |
Z.J. Yin, Z.Q. Wu, F. Lin, et al., Chem. Chin. Lett. 28 (2017) 1167-1171. DOI:10.1016/j.cclet.2017.03.029 |
| [51] |
H.J. Kim, D.R. Whang, J. Gierschner, S.Y. Park, Angew. Chem. Int. Ed. 55 (2016) 15915-15919. DOI:10.1002/anie.201609699 |
| [52] |
H.J. Kim, P.C. Nandajan, J. Gierschner, S.Y. Park, Adv. Funct. Mater. 28 (2017) 1705141. |
| [53] |
X.L. Ni, S. Chen, Y. Yang, Z. Tao, J. Am. Chem. Soc. 138 (2016) 6177-6183. DOI:10.1021/jacs.6b01223 |
| [54] |
S.L. Cai, W.G. Zhang, R.N. Zuckermann, et al., Adv. Mater. 27 (2015) 5762-5770. DOI:10.1002/adma.201500124 |
| [55] |
J. Zhu, C. Yang, C. Lu, et al., Acc. Chem. Res. 51 (2018) 3191-3202. DOI:10.1021/acs.accounts.8b00444 |
| [56] |
X. Feng, A.D. Schlüter, Angew. Chem. Int. Ed. 57 (2018) 13748-13763. DOI:10.1002/anie.201803456 |
| [57] |
R. Sakamoto, K. Takada, T. Pal, et al., Chem. Commun. (Camb.) 53 (2017) 5781-5801. DOI:10.1039/C7CC00810D |
| [58] |
L. Cao, T. Wang, C. Wang, Chin. J. Chem. 36 (2018) 754-764. DOI:10.1002/cjoc.201800144 |
| [59] |
P.J. Waller, F. Gándara, O.M. Yaghi, Acc. Chem. Res. 48 (2015) 3053-3063. DOI:10.1021/acs.accounts.5b00369 |
| [60] |
J. Tian, L. Chen, D.W. Zhang, Y. Liu, Z.T. Li, Chem. Commun. (Camb.) 52 (2016) 6351-6362. DOI:10.1039/C6CC02331B |
| [61] |
J. Tian, H. Wang, D.W. Zhang, Y. Liu, Z.T. Li, Natl. Sci. Rev. 4 (2017) 426-436. DOI:10.1093/nsr/nwx030 |
| [62] |
S.Y. Jiang, X. Zhao, Chin. J. Polym. Sci. 37 (2019) 1-10. DOI:10.1007/s10118-019-2189-0 |
| [63] |
K.D. Zhang, J. Tian, D. Hanifi, et al., J. Am. Chem. Soc. 135 (2013) 17913-17918. DOI:10.1021/ja4086935 |
| [64] |
L. Zhang, T.Y. Zhou, J. Tian, et al., Polym. Chem. 5 (2014) 4715-4721. DOI:10.1039/C4PY00139G |
| [65] |
L. Zhang, Y. Jia, H. Wang, et al., Polym. Chem. 7 (2016) 1861-1865. DOI:10.1039/C5PY02054A |
| [66] |
X. Zhang, C.B. Nie, T.Y. Zhou, et al., Polym. Chem. 6 (2015) 1923-1927. DOI:10.1039/C4PY01669F |
| [67] |
J. Mei, N.L.C. Leung, R.T.K. Kwok, J.W.Y. Lam, B.Z. Tang, Chem. Rev. 115 (2015) 11718-11940. DOI:10.1021/acs.chemrev.5b00263 |
| [68] |
S.Q. Xu, X. Zhang, C.B. Nie, et al., Chem. Commun. (Camb.) 51 (2015) 16417-16420. DOI:10.1039/C5CC05875A |
| [69] |
X.M. Chen, Y.M. Zhang, Y. Liu, Supramol. Chem. 28 (2016) 817-824. DOI:10.1080/10610278.2016.1158406 |
| [70] |
H. Liu, Q. Pan, C. Wu, et al., Mater. Chem. Front. 3 (2019) 1532-1537. DOI:10.1039/C9QM00243J |
| [71] |
Y. Li, Y. Dong, X. Miao, et al., Angew. Chem. Int. Ed. 57 (2018) 729-733. DOI:10.1002/anie.201710553 |
| [72] |
H.J. Lee, H.J. Kim, E.C. Lee, J. Kim, S.Y. Park, Chem. -Asian J. 13 (2018) 390-394. DOI:10.1002/asia.201800020 |
| [73] |
H. Liu, Z. Zhang, Y. Zhao, et al., J. Mater. Chem. B 7 (2019) 1435-1441. |
| [74] |
J. Tian, T.Y. Zhou, S.C. Zhang, et al., Nat. Commun. 5 (2014) 5574. DOI:10.1038/ncomms6574 |
| [75] |
J. Tian, C. Yao, W.L. Yang, et al., Chin. Chem. Lett. 28 (2017) 798-806. DOI:10.1016/j.cclet.2017.01.010 |
| [76] |
C. Yao, J. Tian, H. Wang, et al., Chin. Chem. Lett. 28 (2017) 893-899. DOI:10.1016/j.cclet.2017.01.005 |
| [77] |
B. Yang, X.D. Zhang, J. Li, et al., CCS Chem. 1 (2019) 156-165. DOI:10.31635/ccschem.019.20180011 |
| [78] |
J. Tian, Z.Y. Xu, D.W. Zhang, et al., Nat. Commun. 10 (2016) 11580. |
| [79] |
Y.P. Wu, B. Yang, J. Tian, et al., Chem. Commun. (Camb.) 53 (2017) 13367-13370. DOI:10.1039/C7CC08824H |
| [80] |
Y.P. Wu, M. Yan, Z.Z. Gao, et al., Chin. Chem. Lett. 30 (2019) 1383-1386. DOI:10.1016/j.cclet.2019.03.056 |
| [81] |
S.B. Yu, Q. Qi, B. Yang, et al., Small 14 (2018) 1801037.
|
| [82] |
X.F. Li, S.B. Yu, B. Yang, et al., Sci. China Chem. 61 (2018) 830-835. DOI:10.1007/s11426-018-9234-2 |
| [83] |
M. Yan, X.B. Liu, Z.Z. Gao, et al., Org. Chem. Front. 6 (2019) 1698-1704. DOI:10.1039/C9QO00382G |
| [84] |
Z. Xiang, D. Cao, L. Dai, Polym. Chem. 6 (2015) 1896-1911. DOI:10.1039/C4PY01383B |
| [85] |
P. Puthiaraj, Y.R. Lee, S. Zhang, W.S. Ahn, J. Mater. Chem. A 4 (2016) 16288-16311. DOI:10.1039/C6TA06089G |
| [86] |
R.R. Liang, X. Zhao, Org. Chem. Front. 5 (2018) 3341-3356. DOI:10.1039/C8QO00830B |
| [87] |
X. Chen, K. Geng, R. Liu, et al., Angew. Chem. Int. Ed. 59 (2020) 5050-5091. DOI:10.1002/anie.201904291 |
| [88] |
S. Fischer, J. Schmidt, P. Strauch, A. Thomas, Angew. Chem. Int. Ed. 52 (2013) 12174-12178. DOI:10.1002/anie.201303045 |
| [89] |
Y. Du, H. Yang, J.M. Whiteley, et al., Angew. Chem. Int. Ed. 55 (2016) 1737-1741. DOI:10.1002/anie.201509014 |
| [90] |
H. Ma, B. Liu, B. Li, et al., J. Am. Chem. Soc. 138 (2016) 5897-5903. DOI:10.1021/jacs.5b13490 |
| [91] |
N. Huang, P. Wang, M.A. Addicoat, T. Heine, D. Jiang, Angew. Chem. Int. Ed. 56 (2017) 4982-4986. DOI:10.1002/anie.201611542 |
| [92] |
Z. Li, H. Li, X. Guan, et al., J. Am. Chem. Soc. 139 (2017) 17771-17774. DOI:10.1021/jacs.7b11283 |
| [93] |
J. Roeser, D. Prill, M.J. Bojdys, et al., Nat. Chem. 9 (2017) 977-982. DOI:10.1038/nchem.2771 |
| [94] |
A. Mal, R.K. Mishra, V.K. Praveen, et al., Angew. Chem. Int. Ed. 57 (2018) 8443-8447. DOI:10.1002/anie.201801352 |
| [95] |
K. Murugavel, Polym. Chem. 5 (2014) 5873-5884. DOI:10.1039/C4PY00718B |
| [96] |
A.F. Greene, M.K. Danielson, A.O. Delawder, et al., Chem. Mater. 29 (2017) 9498-9508. DOI:10.1021/acs.chemmater.7b03635 |
| [97] |
K.P. Liles, A.F. Greene, M.K. Danielson, et al., Macromol. Rapid Commun. 39 (2018) 1700781.
|
| [98] |
S.B. Yu, H. Lyu, J. Tian, et al., Polym. Chem. 7 (2016) 3392-3397. DOI:10.1039/C6PY00281A |
| [99] |
G. Das, T. Skorjanc, S.K. Sharma, et al., J. Am. Chem. Soc. 139 (2017) 9558-9565. DOI:10.1021/jacs.7b02836 |
| [100] |
G. Das, T. Skorjanc, S.K. Sharma, et al., ChemNanoMat 4 (2018) 61-65. DOI:10.1002/cnma.201700242 |
| [101] |
L.Z. Peng, P. Liu, Q.Q. Cheng, et al., Chem. Commun. (Camb.) 54 (2018) 4433-4436. DOI:10.1039/C8CC00957K |
| [102] |
P. Samanta, P. Chandra, S. Dutta, A.V. Desaia, S.K. Ghosh, Chem. Sci. 9 (2018) 7874-7881. DOI:10.1039/C8SC02456A |
| [103] |
O. Buyukcakir, S.H. Je, S.N. Talapaneni, D. Kim, A. Coskun, ACS Appl. Mater. Interfaces 9 (2017) 7209-7216. DOI:10.1021/acsami.6b16769 |
| [104] |
O. Buyukcakir, S.H. Je, D.S. Choi, et al., Chem. Commun. (Camb.) 52 (2016) 934-937. DOI:10.1039/C5CC08132G |
| [105] |
G. Chen, X. Huang, Y. Zhang, et al., Chem. Commun. (Camb.) 54 (2018) 12174-12177. DOI:10.1039/C8CC06972G |
| [106] |
G. Das, T. Prakasam, S. Nuryyeva, et al., J. Mater. Chem. A 4 (2016) 15361-15369. DOI:10.1039/C6TA06439F |
| [107] |
T. Skorjanc, D. Shetty, S.K. Sharma, et al., Chem. Eur. J. 24 (2018) 8648-8655. DOI:10.1002/chem.201800623 |
| [108] |
G. Das, S.K. Sharma, T. Prakasam, et al., Commun. Chem. 2 (2019) 106.
|
 2020, Vol. 31
2020, Vol. 31 

