As one of the most widely distributed elements, carbon can exist in various forms: either organic or inorganic compounds in gas, solution, or solid phases. In the form of elementary substance, carbon is revealed to possess several allotropes: the most wellknown forms are graphite and diamond in three dimensions; the low-dimensional new-discovered fullerene [1], carbon nanotubes (CNTs) [2], and graphene [3], as well as the one-dimensional (1D) "old but new" linear carbon chains (LCCs). According to different hybridization states for the C atom, these allotropes can be classified into sp1, sp2, and sp3 hybridizations. Carbon nanomaterials usually possess good electrical conductivity (except diamond), while the electronic band gaps vary greatly among those allotropes. Linear carbon chains (LCCs, also sometimes named as linear carbon, carbon-atom wire, etc.) as 1D sp1-hybridized allotrope of carbon, are either semiconducting (e.g., polyynes) or metallic (e.g., cumulenes). 1D carbon chains can be constructed with two types of bonds: alternative single-triple carbon-carbon bonds to form polyyne or successive double-double bonds to form cumulene, as demonstrated in Fig. 1a. Generally, polyynic structure is more favorable than cumulenic structure, as initially proved through an ab-initio study using Hartree-Fock (HF) theory in 1979 [4]. LCC follows Peierls distortion [5, 6] with bond length alternation (BLA, the bond length difference of single and triple bonds in the center of the chain), thus the cumulene with equidistant C=C bond, which has two degenerate half-filled π-bands, is unstable, whereas the polyyne is more stable due to a longer C-C single bond. Band structure analysis comparing the cumulene and polyyne (Fig. 1b) shows that the valence band of polyyne is fully filled, owing to two electrons provided to each orbital and a half Brillouin zone, and the conduction band is separated by the band gap leading to semi-conductivity. Besides, density functional theory (DFT) calculation by Milani et al. [7] indicated that the cumulene turns out to be a transition state structure reaching a thermodynamically stable state with optimized minimum energy related to polyyne (Fig. 1c). Experimental proof through X-ray crystallography analysis on the bond lengths shows that the Peierls distortion is more significant in longer polyynes [8]. Several reviews on the polyynes and cumulenes are well summarized previously [6, 9-11]. In this review, we focus on the long linear carbon chains with alternative single-triple bonds, i.e., long polyynes. Since various names have been used in the literatures for the carbon chains, we summarized the most frequent appeared names and their corresponding definitions and characters in Table 1.

|
Download:
|
| Fig. 1. Comparing band structures of polyyne (a) and cumulene (b), the red curves show their valence bands. (c) Potential energy diagram of an isolated carbyne as a function of BLA. Copied with permission [11]. Copyright 2016, Royal Society of Chemistry. | |
|
|
Table 1 Definitions and characters for different carbon chains. |
Ideal LCCs are infinite sp-hybridized carbon chains, named as carbyne [12-15]. Many theoretical calculations are based on the infinite carbyne. However, experimentally only finite polyynes or cumulene can be synthesized in the laboratory. For example, polyynes with up to 12 carbon atoms were synthesized [14, 16]. Currently, the longest polyyne containing 44 contiguous acetylenic carbon atoms synthesized via end-capping method was realized by Chalifoux and Tykwinski in 2010 [17]. Remarkably, longer LCCs consisting of more than 100 carbon atoms were grown inside multiwalled carbon nanotubes (MWCNTs) by arc-discharge method in 2003 [18] (Fig. 2c). In 2016, Shi et al. [19] established a route for synthesis of extremely long and stable LCCs containing more than 6000 carbon atoms encapsulated into double-walled carbon nanotubes (DWCNTs) (Fig. 2b). Recently, LCCs were prepared inside single-walled carbon nanotubes (SWCNTs) as well by vacuum discharge method [20, 21] (Fig. 2a). Short LCCs can also be observed when pulling the carbon nanotubes or graphene before breaking [18, 22] as depicted in Figs. 2d and e, although they usually just exist for short time. Small crystal assembled with short polyynes was successfully prepared by laser ablation method when gold was introduced during the synthesis of polyynes [23], as shown in Fig. 2f.

|
Download:
|
| Fig. 2. LCCs inside (a) SWCNTs, (b) DWCNTs, (c) MWCNTs; LCCs derived from (d) carbon nanotubes, (e) graphene; (f) The scheme of crystalline polyynes. | |
UV-vis spectroscopy was used to probe the electronic characteristics of polyynes [8]. However, Raman scattering is a mostfrequent used technic for the polyynic LCCs. The characteristic frequencies for the LCCs are normally located between 1600 cm-1 and 2300 cm-1 [17, 19, 24-27], depending on the length of the LCCs. The longer the polyyne, the lower the frequency [28]. Also, the electron charge polarization could be diminished with increased chain length, resulting in decreasing of the optical energy band gap [14, 29-33]. In addition, the frequency also relies on the environments, e.g., end-capped with chemical groups or encapsulation with carbon nanotubes [25, 34].
This review mainly focuses on introducing polyynic LCCs from both experimental and theoretical aspects. The investigations on the structural and electronic properties of LCCs will be first introduced, and then experimental results mainly on synthesis and characterization are discussed. Finally, possible applications are also briefly presented. Perspectives are present in several sections and in the summary.
2. Properties of linear carbon chainsMost of the properties of LCCs are closely related to the BLA value, e.g., band gap. Therefore, obtaining more accurate BLA is a key research point for calculation and modeling. In the following, we will summary different calculation methods considering the electron-electron, the electron-phonon couplings, etc. for endcapped short polyynes and long polyynic LCCs inside carbon nanotubes.
2.1. Theoretical calculation methods for linear carbon chainsNowadays, the evolved self-consistent field theory on the electronic structure of extended periodic system can calculate the ground-state properties of an isolated molecule accurately [35-37]. Also, calculating the electronic band structure at some fixed geometry based on HF theory was already well accomplished [38-42]. Some modified Huckel theories and complete neglect of differential overlap (CNDO/2) molecular orbital calculations were used to verify the stability of polyynes [43-45]. Later, ab-initio calculations were performed to give numerical results for the equilibrium geometry, vibrational spectra, elastic modulus, band structures, and density of states etc. by using corrected HF approximation with a variety of basis sets based on STO-3 G [4]. A single electron description of the electronic π states in the infinite polyynes shows that the electron possesses an effective internal degeneracy, which leads to an unusual variety of soliton and polaron states [46].
In the 21th century, more reliable results can be obtained with rapid updates of algorithm and hardware. Following the successful observation of the LCCs within the MWCNTs by high-resolution transmission electron microscopy and resonant Raman spectroscopy [18, 47, 48], the Raman peaks at around 1850 cm-1 were assigned to the C-C stretching mode of the LCCs through vibrational calculations. In practice, it is very difficult to accurately simulate the electronic structures of the LCCs due to many reasons [4, 49-57], e.g., obtaining proper BLA, correctly describing electron correlation and electron-phonon coupling [28], the inaccuracy occurred in the system with larger atomic centered basis sets used in the ab-initio calculations [4, 58], etc. Therefore, various theoretical methods and calculation sets led to varied theoretical predictions. For example, Yang et al. [28] concluded the BLA and band gap of polyynes are about 0.13 Å and 2.2 eV, respectively, through the combination of hybrid-DFT scheme with B3LYP// BHHLYP or B3LYP//KMLYP functional. Peach et al. [59] obtained larger BLA value for polyynes using Coulomb-attenuated (CA) CAM-B3LYP functional [60], which is a hybrid exchange-correlation functional proposed in 2004 [60], consisting of a long-range correction based on original B3LYP [61] (Becke gradient-corrected exchange functional [62] and Lee-Yang-Parr correlation functional [63]). In summary, BLA values and HOMO-LUMO gaps for polyynes with 10, 12, 20 carbon atoms and infinite carbyne are listed in Table 2, from which we can conclude that the excitation energy is relatively insensitive to the exchange functional, while the HOMOLUMO gap is more easily affected. The B3LYP results evaluated at the BHHLYP geometry are more consistent with the experimental band gaps [28].
|
|
Table 2 A summary of the BLA values, TDDFT excitation energies, HOMO-LUMO gaps, and longitudinal optical phonon frequencies of hydrogen end-capped polyynes calculated by various methods (N represents the number of the carbon atoms). |
Recently, Mostaani et al. evaluated various theoretical methods used in previous studies [64], for example, HF, nonhybrid DFT, hybrid DFT, MP2, and CCSD(T) calculations [28, 55, 65, 66] to calculate the BLA values of polyynes. In view of the previously reported inconsistent results, they investigated the excitonic band gaps and BLA values of polyynes by employing highly accurate quantum Monte Carlo (QMC) methods. The resulted Peierlsinduced BLA value is 0.136 Å, the DMC quasiparticle gap is 3.61 eV, the static-nucleus DMC singlet excitonic gap is 3.31 eV, and vibrational contributions can reduce the excitonic gap by around 0.1 eV. Besides, the subsequent successful synthesis of LLCCs@CNTs [18, 19] brought the theoretical study focusing on the charge transfer and van der Waals interactions between carbon chains and nanotubes through describing the shifted C-C stretching vibrational mode [25]. Hybrid DFT calculation supported by SCS-MP2 implemented in turbomole [67] using PBE0 functional [68] was employed in the relevant studies. It was mentioned that the disagreement of ab-initio methods for long polyynes can be attributed to the deficiencies of local density functionals or the incomplete description of local and long-range correlation [69-71]. Thus, coupled-cluster CCSD(T) and the DMC methods were applied as solution. The extrapolated BLA by CCSD(T) calculations for the infinite polyynes is 0.125 Å, which is slightly less than that from DMC calculations [64]. While scaling the SCS-MP2 data to fit the CCSD(T) points can yield a G-point frequency at 2075 cm-1 in vacuo [66], which is close to the results from Mostaani et al. [64].
To summarize previous theoretical studies based on LCCs (Table 2), it seems that the HF method obviously overestimates the highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) gap, and the DFT methods with various functionals underestimate the gap; while BHHLYP and CAM-B3LYP give relatively larger but more accurate results. The QMC calculated BLA values and longitudinal optical (LO) phonon frequencies at G-point are significantly larger than the results obtained by the DFT calculations, but more consistent with the experimental results.
2.2. The electronic structure of linear carbon chainFundamentally, the electronic structure of carbon chain is determined by its BLA. Many factors can affect the BLA, for example, the length of the chain, the end-group as termination, the encapsulation by carbon nanotube, etc. Also, the bend effect needs to be considered, since the carbon chain is not always completely straight. Therefore, previously many theoretical studies concentrated on investigating the end-group effect, bending effect, and charge transfer with substrate, etc. [19, 72-80]. In the following, those factors will be discussed in detail.
2.2.1. The length of linear carbon chainPolyyne is unstable due to Peierls distortion, and the Peierls distortion gets more significant when the chain is longer. DFT calculations indicate that Peierls distortion took a main role in affecting the stability of the LCCs, when the number of carbon atoms in a chain is more than fifty [81].
The electric conductivity of molecule usually can be measured by the electronic energy gap or the HOMO-LUMO gap. The BLA value affects the band gap since it varies for the chains with different lengths [82, 83]. Increasing the length of polyyne would lower the HOMO-LUMO gap [29, 84]. However, either the BLA value or the HOMO-LUMO gap of polyyne would never go down to zero no matter how long the chain is due to the Peierls distortion [66].
Phonon dispersion of polyynes with more than 10 carbon atoms was investigated by using oligomer approach [85]. It is shown in effective conjugation coordinate mode that the vibration associated to the triple-bond stretching mode decreases with the increased chain length for even numbered carbon atoms, while being out-ofphase with odd numbered carbon atoms. And for the single carbon bond stretching mode, it is totally opposite. This phenomenon was described as oscillation of the BLA.
The bond types in infinite LCCs are not limited to the conjugated triple bonds in polyyne. When the number of carbon atoms in the LCCs increases to a certain extent that close carbyne system, the BLA will be less oscillated (the vibration of triple bonds stretching mode is related to the LO phonon at Γ-point (q = 0) [86]). To study such a system theoretically, periodical boundary condition should be taken into account by employing first-principle method by eliminating the end-capping effect, thus improving the efficiency of the calculation. It was found that for infinite LCCs, the electronic properties possess similar regularities as for the finite carbon chains [57]. Except the BLA and the HOMO-LUMO gap, the excitation energy is also investigated theoretically for the LCCs. A standard approach to calculate the excitation energies is referred as time-dependent density functional theory [87-89]. The difference between the HOMO-LUMO gap and the excitation energy is called excitonic binding energy, which is a positive value representing the electron-hole interaction.
Long linear carbon chains (LLCCs) ranging from polyyne to carbyne was synthesized inside DWCNTs [24, 90], and the BLA values converge toward a constant value, as suggested by the theoretical prediction long time ago [54, 57, 65]. Such LLCCs can be already referred as carbyne according to the definition given by Sir Harold Walter Kroto.
2.2.2. The end-capped linear carbon chainPolyynes containing 2–44 carbon atoms terminated by various end groups are the most frequently studied carbon chains before the LCCs synthesized inside carbon nanotubes. Many theoretical investigations have been done previously. The ending-group effect was mainly investigated in stabilizing the polyynes. Particularly, studies were also cast on minimizing the end-group effect on the band gap. Hydrogen [33, 91, 92] or methyl [93] caps are the simplest ones for short polyynes, however, they are not good enough for stabilizing long polyynes. tert-Butyl end-caps are better in this case [8].
Basically, introducing bulky end-groups can provide steric hindrance to prevent the cross-linking reactions among the polyyne chains. DFT studies on carbon chains with specific endgroups include hydrogen-, adamantanyl- [27, 94, 95], naphthyl- [96], dinaphthyl- terminations [97], and single-molecule junctions [98], etc.
Recently, Agarwal and Lucotti et al. [34] made a comprehensive investigation on the polyynes consisting of from 8 to 40 carbon atoms with symmetrical (terminated with the same trityl group) or unsymmetrical (terminated with trityl and triisopropylsilyl groups for each end) end-caps. The super trityl bulky end-groups can effectively stabilize polyynes with over 40 carbon atoms in the form of solid powder [17]. Remarkable frequency dispersion (as large as more than 250 cm-1) in Raman line corresponding to the b-mode was observed when changing the length of the polyyne. Also, it was found that the IR/Raman mutual exclusion principle was clearly seen for the polyynes with symmetrical end-caps, and it was weaker for the polyyne with unsymmetrical end-caps.
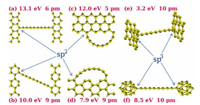
Except normal end-capped chemical groups, sp2 or sp3 carbon terminations, such as graphene, closed-cage clusters, carbon nanotubes, can stabilize the sp carbon chains as well (Fig. 3), and they were investigated by ab-initio and/or experimental studies. It was proposed that the torsional deformation inflicted on the chains affects the BLA, thus tunes the band gap, vibrational frequencies, and magnetization [99, 100]. The calculation showed BLAvalue alone could not completely determine whether the chain is polyynic or cumulenic. Mixed polyynic-cumulenic chains were found with graphene edge and the sp2 terminations made effect on the torsional strain attributed to the plane propagation of terminations through the sequence of π-bonds along the chain direction.
|
Download:
|
| Fig. 3. sp2- and sp3 end-capped carbon chains with binding energies (with respect to the uncapped straight chain plus fully relaxed end-caps) and BLA values. Copied with permission [99]. Copyright 2009, American Physical Society. | |
In addition, carbon chains can also be stabilized by metals, e.g., gold clusters. Synthesis of polyynes using Au as a substrate or catalyst were reported [23, 101]. Surface-enhanced Raman spectroscopic studies on isolated sp-carbon chains presented the interaction between the carbon chains and the silver nanoparticles [102-104]. Tarakeshwar et al. indicated that Au can alter the electron densities of the C≡C bond on both capped and uncapped polyynes by performing ab-initio calculations using B3LYP functional with cc-pVDZ and LANL2DZ basis sets for carbon/ hydrogen and gold, respectively [105]. The result was consistent with previous experimental study [23]. Very recently charge transfers between the chains and the end-groups/metal nanoparticles were discussed, and it was suggested that the role of LCCs played as electron acceptor or donor counter to the silver nanoparticle can be reversed [106].
2.2.3. Linear carbon chains encapsulation by carbon nanotubesAnother route to stabilize the carbon chains is encapsulating the chains inside carbon nanotubes. In the following, the encapsulation effect by the nanotubes on the carbon chains will be discussed.
The structural, electronic, and vibrational properties of confined polyynes with less than 50 carbon atoms and hydrogen capping inside CNTs compared to the free carbon chains were explored through DFT studies [107]. The calculated potential of confined polyyne was about 1 eV, less than that of free carbon chains. Charge transfer between the chains and the nanotubes was confirmed. Additionally, the BLA of the confined polyyne was smaller than that of the free chains due to the charge transfer. Such effect would be different if considering the chirality and diameter of the CNTs [18, 19, 108]. By taking environment and charge transfer into consideration, LLCCs inside narrow inner tubes (0.65– 0.75 nm) will provide a maximal van der Waals interaction. Shi et al. [24] investigated the electronic band gaps of such LLCCs by applying both resonance Raman excitation spectroscopy and DFT calculations. The exitonic energy gaps for LLCCs were in the range of 1.8–2.3 eV, which was about 0.5 eV lower than that of the endcapped polyynes with similar length [25].
2.2.4. Bend effect and charge transfer with environmentsPolyyne was initially assumed to be linear. However, it was demonstrated to be bent in solid state through X-ray crystallographic analyses [109-111]. In addition, it was found that bent polyynes and straight linear cumulenes tended to be formed on less active (e.g., Cu) and active (e.g., Ni, Rh and Ru) metal surfaces, respectively [78]. The imperturbable bending effect may eliminate the influence from end-groups; thus, it turns out to be an intrinsic characteristic of polyyne.
The curvature of polyyne is controlled by the imposed chemical structure confirmed by combing analysis of Raman and IR spectroscopy, as well as DFT simulations [95]. Also, a group of adamantly-end-capped polyynes in both solid and solution states were detailed studied and concluded that the bending effect is inherent [27, 94, 95]. The effective conjugation length of carbon-carbon bonds increases slightly for large end-capped polyyne in comparison with hydrogen end-capped polyyne, causing the bending of the polyyne and inversion symmetry in sequence, thus leading to changes in bond length, bond angle, and band gap [76].
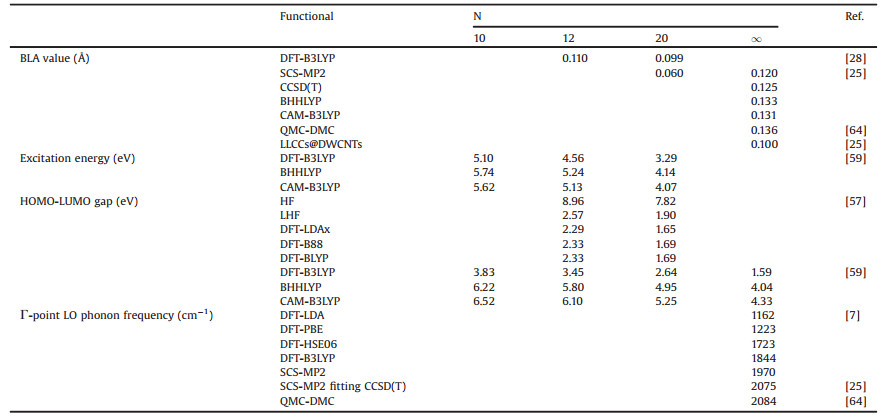
Another example for bent polyyne is kinked carbon chains suggested from theoretical point of view [112-115] (Fig. 4). Twodimensional ordered kinked carbon chains on account of ionassisted condensation [113] (also called Heimann's kink model [114]) generated periodic kinks by shifting inner parts periodically in the plane. Theoretically the kinks can decrease the total energy of the system to stabilize the carbon chains [115]. Experimentally, the phonon vibrations for single chain and inner fragments are weak, but for kinks the phonon vibrations are prominent (1300–1600 cm-1) due to the strong Coulomb interaction between kinked carbon chains.
|
Download:
|
| Fig. 4. sp-Based carbon chains in two-dimensional form ordered by periodic kinks. Copied with permission [112]. Copyright 2017, Elsevier. | |
Carbyne as infinite carbon chain possesses excellent mechanical property as predicted theoretically, suggesting that it may be the strongest material [77]. Based on such great mechanical property, it was found theoretically that a 10% strain not only could not break a chain but enable a strongly increased band gap from 2.6 eV to 4.7 eV, indicating a route to engineer the band gap of the carbon chain [77]. Experimentally, when a carbon chain was pulled out thus created from graphene, the chain under strain behaved semiconducting character, and a so-called ohmic behavior was observed when the chain was relaxed, i.e., without strain [116]. Similarly, metal-insulator transition in carbyne could be induced in carbyne mechanically, meaning that a switch between symmetrical cumulene structure and unsymmetrical polyyne structure can be generated when applying a certain tensile strain on the carbon chain, resulting in a transition from metallic to dielectric state [117].
In addition, Wong et al. developed a Monte Carlo Metropolis algorithm to calculate the macroscopic properties, e.g., thermal expansion and elastic modulus, through simulating microscopic chemical bond distributions and phase transformation in carbon chains [118].
3. Synthesis of carbon chainIn order to tune the band gap down to semiconducting range, synthesizing longer stable polyynes is a key before any real applications.
Polyyne can be decomposed easily when exposure to oxygen and/or water surroundings [26, 119]. As indicated by previous studies on isolated carbon chains in gas phase [120-122] or at very low temperature [123-125], LCCs possess high reactivity to tend to undergo chains cross-linking reaction causing formation of sp2 solid phase [26, 126]. Therefore, it seems that stabilizing the polyyne is of great important. Currently, two main routes were proposed to protect the carbon chains: end-capping the carbon chains or encapsulating the LCCs inside carbon nanotubes. A brief introduction based on these two routes will be present in the following subsections.
3.1. Synthesis of end-capped polyynesPolyynes can be synthesized by organic chemical reactions. The first attempt can date back to 1885 [127], and it was blocked up in isolating tetra-acetylene because of the instability. End-groups, as demonstrated by theoretical studies, have ability to stabilize the polyynes [99]. Experimental synthetic efforts were already paid on preparing various of heteroatom end-capped polyynes, such as hydrogen [14, 16, 128], alkyl [31-33, 93], aryl [129], trialkylsilyl [14] and metal [130-140]. Chalifoux and Tykwinski reported the synthesis of longest polyynes end-capped by trismethyl with up to 44 carbon atoms in the chain [17]. Since this method was widely used and well summarized previously, here we only focus on the methods other than organic chemistry.
Laser ablation was applied to synthesize polyynes efficiently. This method is based on modification of carbon-based solids under high pressure and high temperature [141] and elimination of linear organic substituents [126, 142]. Polyynes end-capped by tert-butyl or trifluoromethyl were grown by using laser-based techniques under gas phase [120]. Moreover, cyano-polyynes were formed through laser ablation of carbon particles in acetonitrile [143]. Tsuji et al. [91] found that hydrogen-capped polyynes with even-numbered carbon atoms from 8 to 16 can be formed by laser ablation of graphite particles suspended in benzene, toluene, or hexane solution. In addition, hydrogen-capped polyynes can be formed by laser ablation of C60 particles suspended in hexane or methanol solution [128]. Instead of organic solvents, water was also used as solvent to prepare short polyynes by laser ablation method [144].
Except synthesis, the mechanism for formation of polyynes by laser ablation was investigated as well. To elucidate the formation mechanism of hydrogen end-capped polyynes in solution, laser ablation of graphite was performed in Ar/propane gas phase, it was found that the length of the chain dependents on the hydrogen source through performing H-abstraction reaction [145]. To determine whether the chemical structures of terminate groups from polyynes could be influenced or not, hydrogen and methyl end-capped polyynes were synthesized by femtosecond laser pulse irradiation directly from liquid toluene, and the type of the end caps were found to be determined by the starting solvent molecule used [146]. Microscopic phase transformation or dissociation of organic molecule is attributed to be the mechanism induced by femtosecond laser irradiation [147-149].
There were also other reported methods, e.g., pyrolysis of starch [150] and carbon rows elimination from graphene nanoflakes through energetic electron irradiation inside a transmission electron microscope (TEM) [22]. Furthermore, LCCs with high thermal stability were formed on the C-terminated β-SiC(100) surface [151]. It was also reported that nature compounds of polyynes can be extracted from some plants and fungi [152-154]. A novel method on producing LCCs by using Pt atoms as termination on a clean graphene surface was proposed [155], the formation, movement on graphene, and bending of LCCs through in-situ transmission electron microscopy were observed. Polyynes were investigated based on oxidative dehydropoly condensation of bisacetylene compounds [156]. Furthermore, it was reported that the stable growth of LCCs without heteroatom end-groups can happen on a cluster-assembled carbon matrix through supersonic carbon cluster beam deposition under room temperature and ultrahigh vacuum [157].
3.2. Linear carbon chains synthesized inside carbon nanotubesCurrently, LLCCs consisting of more than 100 carbon atoms can only be synthesized inside carbon nanotubes. As summarized in Fig. 5, in general, LLCCs can be directly synthesized inside MWCNTs by arc-discharge method, or indirectly grown inside single-, double- and multi-walled carbon nanotubes by posttreatments. In the following, recent progresses are shown in detail.

|
Download:
|
| Fig. 5. Illustration of several synthesis methods for LLCCs inside different CNTs. | |
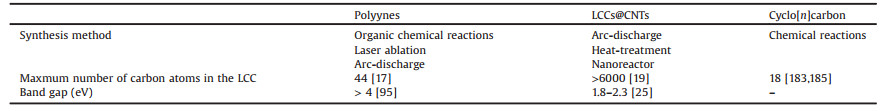
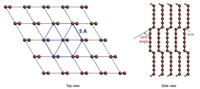
Arc-discharge method was applied to produce different kinds of CNTs by tuning the catalysts in the anode, the current between the anode and the cathode, as well as the filled gases. The LLCCs was first prepared inside MWCNTs by arc-discharge method in 2003 by Zhao et al. [18]. In this method, arc plasma is generated between two carbon electrodes under specific gas background, causing the evaporation of anode and resulting in a deposit on the surface of cathode. The cathode deposit consists of a black area in the center surrounded by a gray shell. The materials in the black area were proved to be LLCCs encapsulated inside MWCNTs. Raman spectra of the materials show Raman features between 1760 cm-1 and 1870 cm-1, attributed to the longitudinal-optical phonon of the LLCCs [24, 90, 158]. The hybrid structure of LLCCs@MWCNTs has been confirmed by transmission electron microscopy in several groups [18, 159]. Especially, in 2015 Andrade et al. observed the cross section of individual LLCCs@MWCNT by using scanning transmission electron microscopy, which presented an unambiguous proof of the encapsulation structure [159].
Nowadays, preparation of the LLCCs@MWCNTs by arc-discharge method can be realized in hydrogen, helium, argon, and even in liquid nitrogen [18, 160-162]. Jinno et al. used H2/N2 mixture to prepare the LLCCs@MWCNTs and found the Raman signal of the LLCCs distinctly decreased with slightly increased ratio of N2 [163]. Kang et al. showed the boron doping in MWCNTs can hamper the growth of LLCCs@MWCNTs [164]. Except the regular way to optimize the arc-discharge method by changing gas and current, Zhang et al. provided a possible way to broaden the range of suitable parameter to produce LLCCs@MWCNTs via enhancing the arc plasma by cooling enhanced hydrogen arcdischarge method [165]. Kim et al. developed an arc apparatus to continually produce MWCNT tapes by atmospheric arc discharge [161], which in principle is able to produce LLCCs@MWCNTs in large scale in future as well.
The growth mechanism of LLCCs@MWCNTs in arc-discharge method is still not completely clarified yet. Generally, it is thought that the growth of LLCCs closely combines the growth of MWCNTs together, because both of the LLCCs and MWCNTs are simultaneously generated within only a few seconds after the stabilization of the arc. Kang et al. suggested a closed-end growth in the arc process that LLCCs are generated from remaining carbon atoms in the innermost tubes and structurally stabilized through van der Waals interaction with the innermost tubes with appropriate diameter [164]. Zhao et al. proposed that the growth mechanism of LLCCs may be similar to that of the smallest carbon nanotubes inside MWCNTs because their preparation condition is quite similar [18].
Note that up to now arc-discharge method can only grow LLCCs@MWCNTs. Catalysts, temperature, and density of carbon species may play important roles, considering the different process among the growth of SWCNTs, DWCNTs and MWCNTs. The growth conditions for the SWCNTs and DWCNTs may be adapted to achieve the growth of the LLCCs@SWCNTs or the LLCCs@DWCNTs, which is indeed a major goal in future.
3.2.2. Heat-treatment methodAs a post treatment, it has been proved that anneal the CNTs at high temperature can produce LLCCs inside CNTs. Endo et al. believed that they produced carbon chains grown between neighbor DWCNTs but not inside the inner tubes of the DWCNTs [166]. The LLCCs@DWCNTs were obtained by annealing SWCNTs or DWCNTs produced by different methods, such as HiPco, eDIPS SWCNTs and CVD-DWCNTs [19, 167, 168]. Sheng et al. proved that this method was as effective as the arc-discharge method [169]. Shi et al. applied the heat-treatment of DWCNTs in high vacuum, resulting in ultra-long LCCs consisting of more than 6000 carbon atoms directly observed by tip-enhanced Raman spectroscopy, which keeps the world's length record since then [19]. In their study, the LLCCs started to grow at around 900 ℃. The relative yield of LLCCs increased with the temperature from 900 ℃ to 1460 ℃. Normally the LLCC growth terminated within 30 min.
LLCCs were first synthesized inside MWCNTs by arc-discharge method, and then inside DWCNTs by heat treatment. The raised question at that time was: Is that possible to stabilize the LLCCs inside SWCNTs? Shi et al. extracted and separated the LLCCs@SWCNTs from the LLCCs@DWCNTs through ultrasonic and ultracentrifugation [21], proving that LLCCs would be stable enough inside SWCNTs.
In addition, not only traditional furnace-based heat-treatment but also other approaches can provide sufficient thermal condition to realize the growth of LLCCs. Ha et al. applied femtosecond laser to treat SWCNT film and it was claimed that LCCs were grown between the walls of CNTs [170]. Toma et al. tried to generate vacuum discharge in the field emission by using CNT film, and signal of LLCCs in the sample after the field emission was observed, suggesting a new method toward preparation of LLCCs@CNTs [20].
For the post-treatment, several issues are in common among the three methods, i.e., heat-treatment, laser irradiation and field emission annealing, which are needed to be clarified. First, what is the carbon source for the formation of the LLCCs and how was the carbon source transformed into LLCCs? The carbon source may come from the amorphous carbon originally inside or surrounded the CNTs and/or even from the CNTs themselves. The diameter distribution of inner tubes in DWCNTs was different after formation of LLCCs. Even new inner tubes can be formed when the starting materials were SWCNTs [171]. Similarly, SWCNTs were changed into DWCNTs or MWCNTs with femtosecond laser irradiation [170]. Therefore, the carbon source could be from both the amorphous carbon and the CNTs. However, direct evidence is still needed to verify the hypothesis. Second, how do the different CNTs affect the synthesis of the LLCCs? Generally, the diameter of SWCNTs or inner tubes of DWCNTs was suggested to be approximately 0.7–1.0 nm, when considering the van der Waals radius of carbon atom [172]. More studies are demanded to understand the relation between the diameter of the CNTs as well as the yield and length of the LLCCs.
3.2.3. Chemical reactions inside CNTsThe post-treatment in principle is physical process that reorganizes the carbon atoms in carbon nanotubes. The hollow structure of carbon nanotube is an ideal nanoreactor to contain small molecules inside and process chemical reaction. The first observation was reported by P. M. Ajayan and S. Iijima that lead wires were prepared by the capillarity-induced filling of MWCNTs [173]. Besides, various materials, such as fullerene, sulfur, water and dye molecules, can be encapsulated inside CNTs [174-177]. The tube provides an excellent condition for the directional selfassembly, controllable reaction, and the growth of metastable structures.
Although this method has been widely used for preparation of novel 1D materials, it is still difficult to obtain LLCCs by chemical reactions inside CNTs. The nanoreactor method requires precise treatment of raw CNTs including cap-opening and high-vacuum degassing. C. Zhao et al. encapsulated polyyne molecules into DWCNTs and those polyynes were then combined into LLCCs via annealing at 1000 ℃ under high vacuum [178]. J. Zhang et al. assembled amantadine inside CNTs and found Raman features related to LLCCs after heating at 600 ℃ [179]. Chimborazo et al. transformed ethanol molecules inside SWCNTs into LLCCs@DWCNTs by using laser annealing in vacuum [180]. Note that this method requires much lower temperature for the growth of the LLCCs than those of the arc-discharge and post-treatment methods, because it is based on the reaction of molecules instead of recrystallization of graphitic structures. More studies in this direction are necessary to enable the synthesis of LLCCs more efficient and controllable.
In short, the above-mentioned methods provide multiple choices for the researchers to obtain LLCCs@CNTs. Different mechanism may enlighten us to better understand the 1D carbon allotrope. However, there are still many challenges in the field, for example, (1) prepare the LLCCs with controllable length, (2) quantify the yield of LLCCs, (3) screen out the LLCC@CNTs from mixed materials with empty CNTs, and (4) extract the LLCCs from the CNTs without damage.
3.3. Synthesis of other types of carbon chainsAs above mentioned, carbon chains can be stabilized by sp2- or sp3 end-capped terminations, as shown in Fig. 3. Experimentally, such carbon chains were indeed prepared when energetic electron irradiate the graphene nanoflakes inside a transmission electron microscope [22]. In principle, in-situ TEM irradiation can be an effective reproducible method to synthesize the LCCs [181]. Moreover, it was found that when stretch a graphene, an ultranarrow ribbon was formed first, then a continuous carbon chain was pulled out of a graphene [182]. Also, such LCCs can be prepared by stretching a carbon nanotube [116].
In 1989, Diederich et al. [183] made efforts on preparing a cyclic carbon clusters with 18 carbon atoms, which was evidenced to be a closed circular polyyne chain [184]. Consequently, self-termination is possible to stabilize the polyyne chain itself without using end-capping or carbon nanotubes, which gives such method a great priority that the carbon chain can be made with only carbon atoms. However, it is too difficult to synthesize polyyne with more carbon atoms. Very recently, a great progress was made that Kaiser et al., successfully prepared a circular chain by manipulating a C24O6 as initio reactant on bilayer NaCl on Cu(111) at 5 K by designing a reaction scheme (Fig. 6), and the obtained circular chain was named as cyclo[18]carbon [185]. Hopefully more circular chains with more carbon atoms can be synthesized in future and easier way to realize the synthesis in large scale would be more expected.
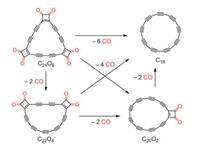
|
Download:
|
| Fig. 6. A reaction process for the formation of cyclo[18]carbon. Copied with permission [185]. Copyright 2019, American Association for the Advancement of Science. | |
Table 3 summarizes the synthesis method, the maximum length and the band gap of different LCCs. Several points can be drawn. First, controllable synthesis of long LCCs with certain length is still challenging. Second, synthesis of stable LCCs in large scale would benefit the application studies in future. The same is for the new explored cyclo[n]carbon.
|
|
Table 3 Summary of the fundunmetal parameters and synthesis methods of the LCCs. |
The last purpose on investigating the sp-based carbon chains is undoubtedly to apply them into various fields. Because of LCCs' excellent electronic, optical, and mechanical properties predicted by the theory, applications using LCCs are with high interest. However, it would be a long way to go, although some researches on possible applications have been reported (mostly theoretically). Currently, fundamental study is still the most important point to solve the perplex challenges, for example, controllably synthesize uniform carbon chains with certain length, synthesize LLCCs as carbyne with narrow band gap, make the LCCs stable enough in different environments, large-scale production of the materials, etc. In this section we will present some interesting recent studies aiming at applications.
It was predicted that LCCs can be functioned as molecular wires to synthesis rotaxanes [186, 187], optical and energy storage devices [26, 188-190], nanoelectronics/spintronic devices [117, 191-194], hydrogen storage devices [195], and even electrode for selective determination of neurotransmitter in the field of biomedicine [196], etc.
Taking the advantage of LCCs' high conductivity, the applications were concentrated on their electrical conductivities in microelectronics. Prazdnikov [197] designed a carbyne transistor being integrable into the silicon technology, which can be scaled up in a rather broad range following the mechanism of inter-chain charge transfer.
The nonlinear optical properties of the LCCs make it as materials for applications such as frequency conversion, optical switching [198]. Photoluminescence and cathodoluminescence of the LCCs could be useful as bio marker [199]. Based on the studies on quantum spin transport in carbon chains [192, 193], LCCs may be used as spin-filter and spin-valve. Tunable transition between magnetic and nonmagnetic states of the LCCs inside armchair SWCNTs can be realized by strain, thus it is possible to use the LCCs in nanodevices, for example mechano-magnetic switch or piezomagnetic sensors [200].
Very recently, the mechanical energy harvesting properties based on a free-standing carbon chain-enriched carbon film prepared via dehydrohalogenation of polyvinylidene fluoride were demonstrated by Krishnamoorthy et al. [201]. A peak power density of 72 nW/cm2 with excellent electromechanical stability was confirmed in the product.
Usually, carbon nanomaterials in different forms can be used as catalysts and/or the framework to support other catalysts, thus, previously they were widely applied in the field of catalysis and batteries [202-204] in the roles for hydrogen evolution reaction [205], CO2/N2/O2 reduction reaction [206-208]. As for LCCs, considering the highly reactivity of the LCCs, it is possible to apply the LCCs as catalysts for similar applications in future.
5. SummaryWe briefly introduce the 1D sp-hybridized polyynic linear carbon chains in different kinds of forms, including its properties, synthesis, and possible applications from both theoretical and experimental aspects. Not only the most important discoveries and stories are presented, but also recent progresses are included in this review.
Lastly, we proposed that several challenges in synthesis should be resolved before any real application, although many predicted advanced properties of the LCCs implies varies promising application directions.
To date, theoretical predictions can give reasonable accurate results on the electronic properties of short or infinite LCCs. However, theoretical studies for the long LCCs with hundreds of carbon atoms are still challenging. In addition, further investigations on the LCCs encapsulated inside CNTs should be carefully performed, and the interaction and charge transfer should be clearly demonstrated in future. From experimental point of view, atomic accurate synthesis of LCCs with controllable length, bond type, dangling bond, doping and crystallization are still impossible. Therefore, further efforts should be made on the precise synthesis. Moreover, large-scale synthesis is also important, since it is much related to the applications of the LCCs in future. Last but not least, using LCCs for possible applications should be carried out in different fields, e.g., catalysis and batteries.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was funded by the National Natural Science Foundation of China (No. 51902353), the Natural Science Foundation of Guangdong Province (No. 2019A1515011227), and the Sun Yat-sen University (Nos. 29000-18841218, 29000-31610028).
| [1] |
H. Kroto, J. Heath, S. O'Brien, R. Curl, R. Smalley, Nature 318 (1985) 162-163. DOI:10.1038/318162a0 |
| [2] |
S. Iijima, Nature 354 (1991) 56-58. DOI:10.1038/354056a0 |
| [3] |
A.K. Geim, K.S. Novoselov, Nat. Mater. 6 (2007) 183-191. DOI:10.1038/nmat1849 |
| [4] |
A. Karpfen, J. Phys, C:Solid State Phys. 12 (1979) 3227-3237. DOI:10.1088/0022-3719/12/16/011 |
| [5] |
R.E. Peierls, Quantum Theory of Solids, Clarendon Press, London, 1996.
|
| [6] |
R. Hoffmann, Angew. Chem. Int. Ed. 26 (1987) 846-878. DOI:10.1002/anie.198708461 |
| [7] |
A. Milani, M. Tommasini, D. Fazzi, et al., J. Raman Spectrosc. 39 (2008) 164-168. DOI:10.1002/jrs.1850 |
| [8] |
W.A. Chalifoux, R. McDonald, M.J. Ferguson, R.R. Tykwinski, Angew. Chem. Int. Ed. 48 (2009) 7915-7919. DOI:10.1002/anie.200902760 |
| [9] |
J.A. Januszewski, R.R. Tykwinski, Chem. Soc. Rev. 43 (2014) 3184-3203. DOI:10.1039/C4CS00022F |
| [10] |
A.L.K. Shi Shun, R.R. Tykwinski, Angew. Chem. Int. Ed. 45 (2006) 1034-1057. DOI:10.1002/anie.200502071 |
| [11] |
C.S. Casari, M. Tommasini, R.R. Tykwinski, A. Milani, Nanoscale 8 (2016) 4414-4435. DOI:10.1039/C5NR06175J |
| [12] |
A. Sladkov, Sov. Sci. Rev. B 3 (1981) 75-110. |
| [13] |
V.V. Korshak, V.I. Kasatochkin, A.M. Sladkov, Y.P. Kudryavtsev, K. Usenbaev, Dokl. Akad. Nauk SSSR 136 (1961) 1342-1344. |
| [14] |
R. Eastmond, T.R. Johnson, D.R.M. Walton, Tetrahedron 28 (1972) 4601-4616. DOI:10.1016/0040-4020(72)80041-3 |
| [15] |
A.G. Whittaker, Nature 276 (1978) 695-696. DOI:10.1038/276695a0 |
| [16] |
E. Kloster-Jensen, Angew. Chem. Int. Ed. 11 (1972) 438-439. DOI:10.1002/anie.197204381 |
| [17] |
W.A. Chalifoux, R.R. Tykwinski, Nat. Chem. 2 (2010) 967-971. DOI:10.1038/nchem.828 |
| [18] |
X. Zhao, Y. Ando, Y. Liu, M. Jinno, T. Suzuki, Phys. Rev. Lett. 90 (2003) 187401. 0.1103/PhysRevLett.90.187401
|
| [19] |
L. Shi, P. Rohringer, K. Suenaga, et al., Nat. Mater. 15 (2016) 634-639. DOI:10.1038/nmat4617 |
| [20] |
S. Toma, K. Asaka, M. Irita, Y. Saito, Surf. Interface Anal. 51 (2019) 131-135. DOI:10.1002/sia.6590 |
| [21] |
L. Shi, K. Yanagi, K. Cao, et al., ACS Nano 12 (2018) 8477-8484. DOI:10.1021/acsnano.8b04006 |
| [22] |
C. Jin, H. Lan, L. Peng, K. Suenaga, S. Iijima, Phys. Rev. Lett. 102 (2009) 205501. DOI:10.1103/PhysRevLett.102.205501 |
| [23] |
B. Pan, J. Xiao, J. Li, et al., Sci. Adv. 1 (2015) e1500857. DOI:10.1126/sciadv.1500857 |
| [24] |
L. Shi, P. Rohringer, M. Wanko, et al., Phys. Rev. Mater. 1 (2017) 075601. DOI:10.1103/PhysRevMaterials.1.075601 |
| [25] |
M. Wanko, S. Cahangirov, L. Shi, et al., Phys. Rev. B 94 (2016) 195422. DOI:10.1103/PhysRevB.94.195422 |
| [26] |
R.B. Heimann, S.E. Evsyukov, L. Kavan, Carbyne and Carbynoid Structures, Springer Science & Business Media, Dordrecht, 1999.
|
| [27] |
A. Lucotti, M. Tommasini, D. Fazzi, et al., J. Raman Spectrosc. 43 (2012) 1293-1298. DOI:10.1002/jrs.3166 |
| [28] |
S. Yang, M. Kertesz, J. Phys. Chem. A 110 (2006) 9771-9774. DOI:10.1021/jp062701+ |
| [29] |
S. Eisler, A.D. Slepkov, E. Elliott, et al., J. Am. Chem. Soc. 127 (2005) 2666-2676. DOI:10.1021/ja044526l |
| [30] |
Q. Zheng, J.C. Bohling, T.B. Peters, et al., Chem. -Eur. J. 12 (2006) 6486-6505. DOI:10.1002/chem.200600615 |
| [31] |
F. Bohlmann, Chem. Ber. 86 (1953) 657-667. DOI:10.1002/cber.19530860519 |
| [32] |
E. Jones, H. Lee, M. Whiting, J. Chem. Soc. (1960) 3483-3489.
|
| [33] |
T.R. Johnson, D.R.M. Walton, Tetrahedron 28 (1972) 5221-5236. DOI:10.1016/S0040-4020(01)88941-9 |
| [34] |
N.R. Agarwal, A. Lucotti, D. Fazzi, et al., J. Raman Spectrosc. 44 (2013) 1398-1410. DOI:10.1002/jrs.4300 |
| [35] |
T. Peacock, R. McWeeny, Proc. Phys. Soc. 74 (1959) 385-394. DOI:10.1088/0370-1328/74/4/301 |
| [36] |
G. Del Re, J. Ladik, G. Biczo, Phys. Rev. 155 (1967) 997-1003. DOI:10.1103/PhysRev.155.997 |
| [37] |
J.M. André, L. Gouverneur, E.G. Leroy, Int. J. Quantum Chem. 1 (1967) 451-461. DOI:10.1002/qua.560010416 |
| [38] |
J.M. André, G. Leroy, Chem. Phys. Lett. 5 (1970) 71-74. DOI:10.1016/0009-2614(70)80004-5 |
| [39] |
M. Kertész, J. Koller, A. Ažman, S. Suhai, Phys. Lett. A 55 (1975) 107-108. |
| [40] |
C. Merkel, J. Ladik, Phys. Lett. A 56 (1976) 395-396. DOI:10.1016/0375-9601(76)90385-6 |
| [41] |
M. Kertész, J. Koller, A. Azman, J. Chem. Phys. 67 (1977) 1180-1186. DOI:10.1063/1.434972 |
| [42] |
S. Suhai, J. Ladik, Solid State Commun. 22 (1977) 227-229. DOI:10.1016/0038-1098(77)90399-4 |
| [43] |
H. Longuet-Higgins, F. Burkitt, Trans. Faraday Soc. 48 (1952) 1077-1084. DOI:10.1039/tf9524801077 |
| [44] |
R. Hoffmann, Tetrahedron 22 (1966) 521-538. DOI:10.1016/0040-4020(66)80020-0 |
| [45] |
I. Stankevich, O. Tomilin, Electronic-Structure of Kabbin Molecules, Mezhdunarodnaya Kniga, Moscow, 1973.
|
| [46] |
M.J. Rice, A.R. Bishop, D.K. Campbell, Phys. Rev. Lett. 51 (1983) 2136-2139. DOI:10.1103/PhysRevLett.51.2136 |
| [47] |
Z. Wang, X. Ke, Z. Zhu, et al., Phys. Rev. B 61 (2000) R2472-R2474. DOI:10.1103/PhysRevB.61.R2472 |
| [48] |
M. Jinno, Y. Ando, S. Bandow, et al., Chem. Phys. Lett. 418 (2006) 109-114. DOI:10.1016/j.cplett.2005.10.089 |
| [49] |
M. Kertesz, J. Koller, A. Azman, J. Chem. Phys. 68 (1978) 2779-2782. DOI:10.1063/1.436070 |
| [50] |
S. Phillpot, M. Rice, A. Bishop, D. Campbell, Phys. Rev. B 36 (1987) 1735-1744. DOI:10.1103/PhysRevB.36.1735 |
| [51] |
M. Springborg, S.L. Drechsler, J. Málek, Phys. Rev. B 41 (1990) 11954-11966. DOI:10.1103/PhysRevB.41.11954 |
| [52] |
E.J. Bylaska, J.H. Weare, R. Kawai, Phys. Rev. B 58 (1998) R7488-R7491. DOI:10.1103/PhysRevB.58.R7488 |
| [53] |
L.U. Horný, N.D.K. Petraco, C. Pak, H.F. Schaefer, J. Am. Chem. Soc. 124 (2002) 5861-5864. DOI:10.1021/ja012014q |
| [54] |
A. Scemama, P. Chaquin, M.C. Gazeau, Y. Bénilan, J. Phys. Chem. A 106 (2002) 3828-3837. DOI:10.1021/jp013043q |
| [55] |
A. Abdurahman, A. Shukla, M. Dolg, Phys. Rev. B 65 (2002) 115106. DOI:10.1103/PhysRevB.65.115106 |
| [56] |
C. Zhang, Z. Cao, H. Wu, Q. Zhang, Int. J. Quantum Chem. 98 (2004) 299-308. DOI:10.1002/qua.20023 |
| [57] |
M. Weimer, W. Hieringer, F.D. Sala, A. Görling, Chem. Phys. 309 (2005) 77-87. DOI:10.1016/j.chemphys.2004.05.026 |
| [58] |
D. Jacquemin, B. Champagne, Int. J. Quantum Chem. 80 (2000) 863-870. DOI:10.1002/1097-461X(2000)80:4/5<863::AID-QUA36>3.0.CO;2-6 |
| [59] |
M.J.G. Peach, E.I. Tellgren, P. Sałek, T. Helgaker, D.J. Tozer, J. Phys. Chem. A 111 (2007) 11930-11935. DOI:10.1021/jp0754839 |
| [60] |
T. Yanai, D.P. Tew, N.C. Handy, Chem. Phys. Lett. 393 (2004) 51-57. DOI:10.1016/j.cplett.2004.06.011 |
| [61] |
Y. Tawada, T. Tsuneda, S. Yanagisawa, T. Yanai, K. Hirao, J. Chem. Phys. 120 (2004) 8425-8433. DOI:10.1063/1.1688752 |
| [62] |
A.D. Becke, J. Chem. Phys. 98 (1993) 5648-5652. DOI:10.1063/1.464913 |
| [63] |
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37 (1988) 785-789. DOI:10.1103/PhysRevB.37.785 |
| [64] |
E. Mostaani, B. Monserrat, N.D. Drummond, C.J. Lambert, Phys. Chem. Chem. Phys. 18 (2016) 14810-14821. DOI:10.1039/C5CP07891A |
| [65] |
T.D. Poulsen, K.V. Mikkelsen, J.G. Fripiat, D. Jacquemin, B. Champagne, J. Chem. Phys. 114 (2001) 5917-5922. DOI:10.1063/1.1353550 |
| [66] |
C.D. Zeinalipour-Yazdi, D.P. Pullman, J. Phys. Chem. B 112 (2008) 7377-7386. DOI:10.1021/jp800302s |
| [67] |
V. TURBOMOLE, A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, TURBOMOLE GmbH since 2007, 2010.
|
| [68] |
J.P. Perdew, M. Ernzerhof, K. Burke, J. Chem. Phys. 105 (1996) 9982-9985. DOI:10.1063/1.472933 |
| [69] |
T. Körzdörfer, R.M. Parrish, J.S. Sears, C.D. Sherrill, J.L. Brédas, J. Chem. Phys. 137 (2012) 124305.
|
| [70] |
D. Jacquemin, C. Adamo, J. Chem. Theory Comput. 7 (2011) 369-376. DOI:10.1021/ct1006532 |
| [71] |
M. Wykes, N.Q. Su, X. Xu, C. Adamo, J.C. Sancho-García, J. Chem. Theory Comput. 11 (2015) 832-838. DOI:10.1021/ct500986b |
| [72] |
W. Luo, W. Windl, Carbon 47 (2009) 367-383. DOI:10.1016/j.carbon.2008.10.017 |
| [73] |
Y.H. Hu, Phys. Lett. A 373 (2009) 3554-3557. DOI:10.1016/j.physleta.2009.07.067 |
| [74] |
S. Cahangirov, M. Topsakal, S. Ciraci, Phys. Rev. B 82 (2010) 195444. DOI:10.1103/PhysRevB.82.195444 |
| [75] |
Z. Lin, X. Ning, Europhys. Lett. 95 (2011) 47012. DOI:10.1209/0295-5075/95/47012 |
| [76] |
Y.H. Hu, J. Phys. Chem. C 115 (2011) 1843-1850. DOI:10.1021/jp111851u |
| [77] |
M. Liu, V.I. Artyukhov, H. Lee, F. Xu, B.I. Yakobson, ACS Nano 7 (2013) 10075-10082. DOI:10.1021/nn404177r |
| [78] |
Q. Yuan, F. Ding, Nanoscale 6 (2014) 12727-12731. DOI:10.1039/C4NR03757J |
| [79] |
A. Timoshevskii, S. Kotrechko, Y. Matviychuk, Phys. Rev. B 91 (2015) 245434. DOI:10.1103/PhysRevB.91.245434 |
| [80] |
C.B. Cannella, N. Goldman, J. Phys. Chem. C 119 (2015) 21605-21611. DOI:10.1021/acs.jpcc.5b03781 |
| [81] |
S. Yang, M. Kertesz, J. Phys. Chem. A 112 (2008) 146-151. DOI:10.1021/jp076805b |
| [82] |
M. Kertesz, C.H. Choi, S. Yang, Chem. Rev. 105 (2005) 3448-3481. DOI:10.1021/cr990357p |
| [83] |
A. Milani, M. Tommasini, G. Zerbi, J. Raman Spectrosc. 40 (2009) 1931-1934. DOI:10.1002/jrs.2342 |
| [84] |
T. Gibtner, F. Hampel, J.P. Gisselbrecht, A. Hirsch, Chem.-Eur. J. 8 (2002) 408-432. DOI:10.1002/1521-3765(20020118)8:2<408::AID-CHEM408>3.0.CO;2-L |
| [85] |
R. Zbinden, Infrared Spectroscopy of High Polymers, Academic Press, New York, 1964.
|
| [86] |
M. Tommasini, D. Fazzi, A. Milani, et al., J. Phys. Chem. A 111 (2007) 11645-11651. DOI:10.1021/jp0757006 |
| [87] |
E. Gross, J. Dobson, M. Petersilka, Density functional theory, in: R.F. Nalewajski (Ed.), Topics in Current Chemistry, Springer, Berlin, 1996, pp. 81-172.
|
| [88] |
D.P. Cong, Recent Advances in Density Functional Methods, World Scientific, Singapore, 1995.
|
| [89] |
R. Bauernschmitt, R. Ahlrichs, Chem. Phys. Lett. 256 (1996) 454-464. DOI:10.1016/0009-2614(96)00440-X |
| [90] |
S. Heeg, L. Shi, L.V. Poulikakos, T. Pichler, L. Novotny, Nano Lett. 18 (2018) 5426-5431. DOI:10.1021/acs.nanolett.8b01681 |
| [91] |
M. Tsuji, T. Tsuji, S. Kuboyama, et al., Chem. Phys. Lett. 355 (2002) 101-108. DOI:10.1016/S0009-2614(02)00192-6 |
| [92] |
F. Cataldo, Tetrahedron Lett. 45 (2004) 141-144. DOI:10.1016/j.tetlet.2003.10.100 |
| [93] |
C. Cook, E. Jones, M. Whiting, J. Chem. Soc. (1952) 2883-2891.
|
| [94] |
A. Lucotti, M. Tommasini, D. Fazzi, et al., J. Am. Chem. Soc. 131 (2009) 4239-4244. DOI:10.1021/ja078198b |
| [95] |
A. Lucotti, M. Tommasini, W.A. Chalifoux, et al., J. Raman Spectrosc. 43 (2012) 95-101. DOI:10.1002/jrs.2992 |
| [96] |
F. Cataldo, L. Ravagnan, E. Cinquanta, et al., J. Phys. Chem. B 114 (2010) 14834-14841. DOI:10.1021/jp104863v |
| [97] |
E. Cinquanta, L. Ravagnan, I.E. Castelli, et al., J. Chem. Phys. 135 (2011) 194501. DOI:10.1063/1.3660211 |
| [98] |
S. Ballmann, W. Hieringer, D. Secker, et al., ChemPhysChem 11 (2010) 2256-2260. DOI:10.1002/cphc.200900974 |
| [99] |
L. Ravagnan, N. Manini, E. Cinquanta, et al., Phys. Rev. Lett. 102 (2009) 245502. DOI:10.1103/PhysRevLett.102.245502 |
| [100] |
G. Onida, N. Manini, L. Ravagnan, et al., Phys. Status Solidi B 247 (2010) 2017-2021. DOI:10.1002/pssb.200983946 |
| [101] |
K.H. Xue, S.P. Chen, L.X. Wang, et al., Chem. Phys. Lett. 469 (2009) 284-288. DOI:10.1016/j.cplett.2008.12.075 |
| [102] |
A. Lucotti, M. Tommasini, M.D. Zoppo, et al., Chem. Phys. Lett. 417 (2006) 78-82. DOI:10.1016/j.cplett.2005.10.016 |
| [103] |
A. Lucotti, C.S. Casari, M. Tommasini, et al., Chem. Phys. Lett. 478 (2009) 45-50. DOI:10.1016/j.cplett.2009.06.030 |
| [104] |
A. Milani, A. Lucotti, V. Russo, et al., J. Phys. Chem. C 115 (2011) 12836-12843. DOI:10.1021/jp203682c |
| [105] |
P. Tarakeshwar, P.R. Buseck, H.W. Kroto, J. Phys. Chem. Lett. 7 (2016) 1675-1681. DOI:10.1021/acs.jpclett.6b00671 |
| [106] |
A. Milani, V. Barbieri, A. Facibeni, et al., Sci. Rep. 9 (2019) 1648. DOI:10.1038/s41598-018-38367-9 |
| [107] |
X. Fan, L. Liu, J. Lin, Z. Shen, J.L. Kuo, ACS Nano 3 (2009) 3788-3794. DOI:10.1021/nn901090e |
| [108] |
A. Cupolillo, M. Castriota, E. Cazzanelli, et al., J. Raman Spectrosc. 39 (2008) 147-152. DOI:10.1002/jrs.1871 |
| [109] |
S. Szafert, J. Gladysz, Chem. Rev. 106 (2006) PR1-PR33. DOI:10.1021/cr040095d |
| [110] |
W.F. Maier, G.C. Lau, A.B. McEwen, J. Am. Chem. Soc. 107 (1985) 4724-4731. DOI:10.1021/ja00302a021 |
| [111] |
C. Santiago, K. Houk, G.J. DeCicco, L.T. Scott, J. Am. Chem. Soc. 100 (1978) 692-696. DOI:10.1021/ja00471a005 |
| [112] |
E.A. Buntov, A.F. Zatsepin, M.B. Guseva, Y.S. Ponosov, Carbon 117 (2017) 271-278. DOI:10.1016/j.carbon.2017.03.010 |
| [113] |
V. Babaev, M. Guseva, Ion-assisted condensation of carbon, in: R.B. Heimann, S.E. Evsyukov, L. Kavan (Eds.), Eds.), Carbyne and Carbynoid Structures, Springer, Dordrecht, 1999, pp. 159-171.
|
| [114] |
R. Heimann, J. Kleiman, N. Salansky, Nature 306 (1983) 164-167. DOI:10.1038/306164a0 |
| [115] |
V. Babaev, M. Guseva, V. Khvostov, N. Novikov, P. Flood, Carbon material with a highly ordered linear-chain structure, in: F. Cataldo (Ed.), Polyynes: Synthesis, Properties, and Applications, CRC Press, Boca Raton, 2005, pp. 219-252.
|
| [116] |
A. La Torre, A. Botello-Mendez, W. Baaziz, J.C. Charlier, F. Banhart, Nat. Commun. 6 (2015) 6636.
|
| [117] |
V.I. Artyukhov, M. Liu, B.I. Yakobson, Nano Lett. 14 (2014) 4224-4229. DOI:10.1021/nl5017317 |
| [118] |
C. Wong, E. Buntov, V. Rychkov, M. Guseva, A. Zatsepin, Carbon 114 (2017) 106-110. DOI:10.1016/j.carbon.2016.12.009 |
| [119] |
J. Kastner, H. Kuzmany, L. Kavan, F. Dousek, J. Kürti, Macromolecules 28 (1995) 344-353. DOI:10.1021/ma00105a048 |
| [120] |
R.J. Lagow, J.J. Kampa, H.C. Wei, et al., Science 267 (1995) 362-367. DOI:10.1126/science.267.5196.362 |
| [121] |
T. Pino, H. Ding, F. Güthe, J.P. Maier, J. Chem. Phys. 114 (2001) 2208-2212. DOI:10.1063/1.1338530 |
| [122] |
M.C. McCarthy, P. Thaddeus, Chem. Soc. Rev. 30 (2001) 177-185. DOI:10.1039/b006648f |
| [123] |
W. Krätschmer, N. Sorg, D.R. Huffman, Surf. Sci. 156 (1985) 814-821. DOI:10.1016/0039-6028(85)90253-5 |
| [124] |
A.K. Ott, G.A. Rechtsteiner, C. Felix, et al., J. Chem. Phys. 109 (1998) 9652-9655. DOI:10.1063/1.477632 |
| [125] |
J. Szczepanski, J. Fuller, S. Ekern, M. Vala, Spectrochim, Acta, Part A 57 (2001) 775-786. DOI:10.1016/S1386-1425(00)00443-1 |
| [126] |
L. Kavan, Chem. Rev. 97 (1997) 3061-3082. DOI:10.1021/cr960003n |
| [127] |
A. Baeyer, Ber. Dtsch. Chem. Ges. 18 (1885) 2269-2281. DOI:10.1002/cber.18850180296 |
| [128] |
M. Tsuji, S. Kuboyama, T. Matsuzaki, T. Tsuji, Carbon 41 (2003) 2141-2148. DOI:10.1016/S0008-6223(03)00241-0 |
| [129] |
M. Nakagawa, S. Akiyama, K. Nakasuji, K. Nishimoto, Tetrahedron 27 (1971) 5401-5418. DOI:10.1016/S0040-4020(01)91706-5 |
| [130] |
U.H. Bunz, Angew. Chem. Int. Ed. 35 (1996) 969-971. DOI:10.1002/anie.199609691 |
| [131] |
M.I. Bruce, P.J. Low, Adv. Organomet. Chem. 50 (2004) 180-444. |
| [132] |
M.I. Bruce, Coord. Chem. Rev. 166 (1997) 91-119. DOI:10.1016/S0010-8545(97)00004-0 |
| [133] |
N.J. Long, C.K. Williams, Angew. Chem. Int. Ed. 42 (2003) 2586-2617. DOI:10.1002/anie.200200537 |
| [134] |
A.B. Antonova, M.I. Bruce, B.G. Ellis, et al., Chem. Commun. 8 (2004) 960-961. DOI:10.1039/b315854n |
| [135] |
V.W.W. Yam, K.M.C. Wong, N. Zhu, Angew. Chem. Int. Ed. 42 (2003) 1400-1403. DOI:10.1002/anie.200390360 |
| [136] |
G.L. Xu, G. Zou, Y.H. Ni, et al., J. Am. Chem. Soc. 125 (2003) 10057-10065. DOI:10.1021/ja035434j |
| [137] |
W. Lu, H.F. Xiang, N. Zhu, C.M. Che, Organometallics 21 (2002) 2343-2346. DOI:10.1021/om011087f |
| [138] |
A. Sakurai, M. Akita, Y. Moro-oka, Organometallics 18 (1999) 3241-3244. DOI:10.1021/om990266i |
| [139] |
M. Akita, M.C. Chung, A. Sakurai, et al., Organometallics 16 (1997) 4882-4888. DOI:10.1021/om970538m |
| [140] |
R. Dembinski, T. Bartik, B. Bartik, M. Jaeger, J. Gladysz, J. Am. Chem. Soc. 122 (2000) 810-822. DOI:10.1021/ja992747z |
| [141] |
R.B. Heimann, Diamond Relat. Mater. 3 (1994) 1151-1157. DOI:10.1016/0925-9635(94)90161-9 |
| [142] |
L. Kavan, J. Kastner, Carbon 32 (1994) 1533-1536. DOI:10.1016/0008-6223(94)90149-X |
| [143] |
T. Wakabayashi, M. Saikawa, Y. Wada, T. Minematsu, Carbon 50 (2012) 47-56. DOI:10.1016/j.carbon.2011.07.053 |
| [144] |
G. Compagnini, V. Mita, R.S. Cataliotti, L. D'Urso, O. Puglisi, Carbon 12 (2007) 2456-2458. DOI:10.1016/j.carbon.2007.07.002 |
| [145] |
Y. Taguchi, H. Endo, Y. Abe, et al., Carbon 94 (2015) 124-128. DOI:10.1016/j.carbon.2015.06.058 |
| [146] |
A. Ramadhan, M. Wesolowski, T. Wakabayashi, et al., Carbon 118 (2017) 680-685. DOI:10.1016/j.carbon.2017.03.096 |
| [147] |
A. Hu, M. Rybachuk, Q.B. Lu, W. Duley, Appl. Phys. Lett. 91 (2007) 131906. DOI:10.1063/1.2793628 |
| [148] |
A. Hu, Q.B. Lu, W.W. Duley, M. Rybachuk, J. Chem. Phys. 126 (2007) 154705. DOI:10.1063/1.2727450 |
| [149] |
A. Hu, J. Sanderson, A. Zaidi, et al., Carbon 46 (2008) 1823-1825. DOI:10.1016/j.carbon.2008.07.036 |
| [150] |
K.H. Xue, F.F. Tao, W. Shen, et al., Chem. Phys. Lett. 385 (2004) 477-480. DOI:10.1016/j.cplett.2004.01.007 |
| [151] |
V. Derycke, P. Soukiassian, A. Mayne, G. Dujardin, J. Gautier, Phys. Rev. Lett. 81 (1998) 5868-5871. DOI:10.1103/PhysRevLett.81.5868 |
| [152] |
V. Vil'yams, V. Smimov, V. Gol'mov, Obsch. Khim 5 (1935) 1195-1204. |
| [153] |
J.D. Bu'Lock, Q. Rev, Chem. Soc. 10 (1956) 371-394. |
| [154] |
G. Nakaminami, J. Synth, Org. Chem Jpn. 21 (1963) 751-765. DOI:10.5059/yukigoseikyokaishi.21.751 |
| [155] |
E. Kano, M. Takeguchi, J.I. Fujita, A. Hashimoto, Carbon 80 (2014) 382-386. DOI:10.1016/j.carbon.2014.08.077 |
| [156] |
Y.P. Kudryavtsev, Oxidative dehydropolycondensation-a new method to synthesize polymers with triple bonds, in: V.V. Korshak (Ed.), Progress in Polymer Chemistry, Nauka, Moscow, 1969, pp. 87-112.
|
| [157] |
C.S. Casari, A.L. Bassi, L. Ravagnan, et al., Phys. Rev. B 69 (2004) 075422. DOI:10.1103/PhysRevB.69.075422 |
| [158] |
D. Nishide, T. Wakabayashi, T. Sugai, et al., J. Phys. Chem. C 111 (2007) 5178-5183. DOI:10.1021/jp0686442 |
| [159] |
N. Andrade, T. Vasconcelos, C. Gouvea, et al., Carbon 90 (2015) 172-180. DOI:10.1016/j.carbon.2015.04.001 |
| [160] |
E. Cazzanelli, M. Castriota, L. Caputi, et al., Phys. Rev. B 75 (2007) 121405. DOI:10.1103/PhysRevB.75.121405 |
| [161] |
Y.A. Kim, H. Muramatsu, T. Hayashi, M. Endo, Carbon 50 (2012) 4588-4595. DOI:10.1016/j.carbon.2012.05.044 |
| [162] |
V. Scuderi, S. Scalese, S. Bagiante, et al., Carbon 47 (2009) 2134-2137. DOI:10.1016/j.carbon.2009.04.010 |
| [163] |
M. Jinno, S. Bandow, Y. Ando, Chem. Phys. Lett. 398 (2004) 256-259. DOI:10.1016/j.cplett.2004.09.064 |
| [164] |
C.S. Kang, K. Fujisawa, Y.I. Ko, et al., Carbon 107 (2016) 217-224. DOI:10.1016/j.carbon.2016.05.069 |
| [165] |
Y. Zhang, J. Zhao, Y. Fang, Y. Liu, X. Zhao, Nanoscale 10 (2018) 17824-17833. DOI:10.1039/C8NR05465G |
| [166] |
M. Endo, Y.A. Kim, T. Hayashi, et al., Small 2 (2006) 1031-1036. DOI:10.1002/smll.200600087 |
| [167] |
L. Shi, P. Rohringer, P. Ayala, T. Saito, T. Pichler, Phys. Status Solidi B 250 (2013) 2611-2615. DOI:10.1002/pssb.201300148 |
| [168] |
L. Shi, L. Sheng, L. Yu, et al., Nano Res. 4 (2011) 759-766. DOI:10.1007/s12274-011-0132-y |
| [169] |
L. Sheng, A. Jin, L. Yu, et al., Mater. Lett. 81 (2012) 222-224. DOI:10.1016/j.matlet.2012.04.140 |
| [170] |
J. Ha, H.Y. Jung, J. Hao, et al., Nanoscale 9 (2017) 16627-16631. DOI:10.1039/C7NR05883G |
| [171] |
L. Shi, J. Wei, K. Yanagi, et al., Nanoscale 10 (2018) 21254-21261. DOI:10.1039/C8NR06925E |
| [172] |
Y. Liu, R. Jones, X. Zhao, Y. Ando, Phys. Rev. B 68 (2003) 125413. DOI:10.1103/PhysRevB.68.125413 |
| [173] |
P.M. Ajayan, Nature 361 (1993) 333-334. DOI:10.1038/361333a0 |
| [174] |
A.N. Khlobystov, D.A. Britz, A. Ardavan, G.A.D. Briggs, Phys. Rev. Lett. 92 (2004) 245507. DOI:10.1103/PhysRevLett.92.245507 |
| [175] |
H. Kuzmany, L. Shi, J. Kürti, et al., Phys. Status Solidi RRL 11 (2017) 1700158. DOI:10.1002/pssr.201700158 |
| [176] |
T. Fujimori, A. Morelos-Gómez, Z. Zhu, et al., Nat. Commun. 4 (2013) 2162. DOI:10.1038/ncomms3162 |
| [177] |
S. Cambré, J. Campo, C. Beirnaert, et al., Nat. Nanotechnol. 10 (2015) 248-252. DOI:10.1038/nnano.2015.1 |
| [178] |
C. Zhao, R. Kitaura, H. Hara, S. Irle, H. Shinohara, J. Phys, Chem. C 115 (2011) 13166-13170. |
| [179] |
J. Zhang, Y. Feng, H. Ishiwata, et al., ACS Nano 6 (2012) 8674-8683. DOI:10.1021/nn303461q |
| [180] |
J. Chimborazo, T. Saito, T. Pichler, L. Shi, P. Ayala, Appl. Phys. Lett. 115 (2019) 103102. DOI:10.1063/1.5095679 |
| [181] |
G. Casillas, A. Mayoral, M. Liu, et al., Carbon 66 (2014) 436-441. DOI:10.1016/j.carbon.2013.09.019 |
| [182] |
A. Chuvilin, J.C. Meyer, G. Algara-Siller, U. Kaiser, New J. Phys. 11 (2009) 083019. DOI:10.1088/1367-2630/11/8/083019 |
| [183] |
F. Diederich, Y. Rubin, C.B. Knobler, et al., Science 245 (1989) 1088-1090. DOI:10.1126/science.245.4922.1088 |
| [184] |
D.A. Plattner, K. Houk, J. Am. Chem. Soc. 117 (1995) 4405-4406. DOI:10.1021/ja00120a026 |
| [185] |
K. Kaiser, L.M. Scriven, F. Schulz, et al., Science 365 (2019) 1299-1301. DOI:10.1126/science.aay1914 |
| [186] |
L.D. Movsisyan, D.V. Kondratuk, M. Franz, et al., Org. Lett. 14 (2012) 3424-3426. DOI:10.1021/ol301392t |
| [187] |
N. Weisbach, Z. Baranová, S. Gauthier, J.H. Reibenspies, J.A. Gladysz, Chem. Commun. 48 (2012) 7562-7564. DOI:10.1039/c2cc33321j |
| [188] |
Y. Tobe, T. Wakabayashi, Acetylene-based carbon allotropes, in: F. Diederich, P.J. Stang, R.R. Tykwinski (Eds.), Eds.), Acetylene Chemistry: Chemistry, Biology, and Material Science, John Wiley & Sons, Weinheim, 2004, pp. 387-426.
|
| [189] |
A.D. Slepkov, F.A. Hegmann, S. Eisler, E. Elliott, R.R. Tykwinski, J. Chem. Phys. 120 (2004) 6807-6810. DOI:10.1063/1.1707011 |
| [190] |
S. Shiraishi, T. Kobayashi, A. Oya, Chem. Lett. 34 (2005) 1678-1679. DOI:10.1246/cl.2005.1678 |
| [191] |
K.H. Khoo, J. Neaton, Y.W. Son, M.L. Cohen, S.G. Louie, Nano Lett. 8 (2008) 2900-2905. DOI:10.1021/nl8017143 |
| [192] |
Z. Zanolli, G. Onida, J.C. Charlier, ACS Nano 4 (2010) 5174-5180. DOI:10.1021/nn100712q |
| [193] |
M. Zeng, L. Shen, Y. Cai, Z. Sha, Y. Feng, Appl. Phys. Lett. 96 (2010) 042104. DOI:10.1063/1.3299264 |
| [194] |
B. Akdim, R. Pachter, ACS Nano 5 (2011) 1769-1774. DOI:10.1021/nn102403j |
| [195] |
P.B. Sorokin, H. Lee, L.Y. Antipina, A.K. Singh, B.I. Yakobson, Nano Lett. 11 (2011) 2660-2665. DOI:10.1021/nl200721v |
| [196] |
K.H. Xue, F.F. Tao, W. Xu, S.Y. Yin, J.M. Liu, J. Electroanal. Chem. 578 (2005) 323-329. DOI:10.1016/j.jelechem.2005.01.027 |
| [197] |
Y.E. Prazdnikov, J. Mod. Phys. 2 (2011) 845-848. DOI:10.4236/jmp.2011.28100 |
| [198] |
C. Ma, J. Xiao, G. Yang, J. Mater. Chem. C 4 (2016) 4692-4698. DOI:10.1039/C6TC00648E |
| [199] |
J. Xiao, J. Li, G. Yang, Small 13 (2017) 1603495. DOI:10.1002/smll.201603495 |
| [200] |
B. Xu, J. Lin, Y. Feng, Appl. Phys. Lett. 96 (2010) 163105. DOI:10.1063/1.3397995 |
| [201] |
K. Krishnamoorthy, V.K. Mariappan, P. Pazhamalai, S. Sahoo, S.J. Kim, Nano Energy 59 (2019) 453-463. DOI:10.1016/j.nanoen.2019.02.041 |
| [202] |
J. Liu, T. He, Q. Wang, et al., J. Mater. Chem. A 7 (2019) 12451-12456. DOI:10.1039/C9TA02264C |
| [203] |
H. Shi, W. Lv, C. Zhang, et al., Adv. Funct. Mater. 28 (2018) 1800508. DOI:10.1002/adfm.201800508 |
| [204] |
J. Han, D. Kong, W. Lv, et al., Nat. Commun. 9 (2018) 1-9. DOI:10.1038/s41467-017-02088-w |
| [205] |
Y. Lei, Y. Wang, Y. Liu, et al., Angew. Chem. Int. Ed. (2020), doi: http://dx.doi.org/10.1002/ange.201914647.
|
| [206] |
Q. Wang, Y. Lei, D. Wang, Y. Li, Energ. Environ. Sci. 12 (2019) 1730-1750. DOI:10.1039/C8EE03781G |
| [207] |
L. Liu, Z.Q. Niu, J. Chen, Chin. Chem. Lett. 29 (2018) 571-581. DOI:10.1016/j.cclet.2018.01.013 |
| [208] |
L. Chen, X. Xu, W. Yang, J. Jia, Chin. Chem. Lett. 31 (2020) 626-634. DOI:10.1016/j.cclet.2019.08.008 |
 2020, Vol. 31
2020, Vol. 31