b Department of Clinical Laboratory, Yizheng Hospital of Nanjing Drum Tower Hospital Group, Yizheng 211900, China;
c Department of Clinical Laboratory, the Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing 210008, China;
d State Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, National Demonstration Center for Experimental Biomedical Engineering Education(Southeast University), Southeast University, Nanjing 210096, China;
e Economical Forest Cultivation and Utilization of 2011 Collaborative Innovation Center in Hunan Province, Hunan Key Laboratory of Biomedical Nanomaterials and Devices, Hunan University of Technology, Zhuzhou 412007, China
Extracellular vesicles (EVs) are heterogeneous vesicles shed by a variety of mammalian cells, especially dividing cancer cells, as well as normal cells [1-5]. Biofluids contain a large number of EVs that can shuttle between parental cells and other cells. Initially, under-appreciated as "cell dust'" and as a mechanism to dispose of cellular components, EVs are now considered abundant and stable sources of biomacromolecule, including proteins, mRNA/miRNA and DNA, thus serving as cellular surrogates [6-10]. These biomacromolecules are key component of the tumor microenvironment and participate in adaptation and regulation of immunity, involved in the regulation of pathological angiogenesis, including tumor angiogenesis, tumor growth and metastasis, which verify EVs have the significant advantages in cancer monitoring. Tumorassociated vesicles can be used as effective surrogate biomarkers to define tumor type, stage and underneath mutations, as well as to monitor treatment response [11-15]. Specifically, EVs provide a minimally invasive avenue for obtaining information from tumor cells, and can be longitudinally monitored by repeated sampling [14].
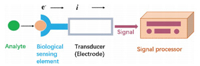
As EVs are too small to be detected, it is necessary to find more sensitive methods for their detection [8]. Conventional detection techniques such as Western blotting and enzyme-linked immunosorbent assays (ELISAs) are commonly detection technologies. However, they require large amounts of samples, and become impractical for clinical research needs, notably serial analyses, large patient cohorts, or limited specimens in biorepositories [16-18]. Electrochemical biosensors are a subclass of chemical sensors that combine sensitivity, as indicated by low detection limits and real-time, of electrochemical transducers with high specificity of biological recognition processes [19-21]. These devices contain biometric elements (enzymes, proteins, antibodies, nucleic acids, cells, tissues or receptors) that, upon selective reaction with analyte of interest, produce electrical signals related to the concentration of the analyte being studied (Fig. 1) [22].
|
Download:
|
| Fig. 1. Illustration of electrochemical biosensor. | |
This article reviews the biogenesis of EVs and the recently emerged electrochemically based methods for quantitative detection of EVs. Highlight on pertinent applications of EVs in the diagnosis and treatment of diseases, such as screening of EVs as potential tumor markers.
2. EVs biogenesisEVs include exomeres, microvesicles, exosomes, apoptotic bodies, blebs and argosomes, and are classified by size, content, origin and function [23], as shown in Table 1. Exomeres are a recently discovered extracellular nanoparticles with unknown biological function, rich in argonaute and amyloid precursor protein [24, 25]. Exosomes were first proposed by Trams et al. in the 1980s. They were first discovered in the supernatant obtained from sheep red blood cells, cultured in vitro, with vesicle structures from 40 nm to 200 nm. The exosomes are composed of a phospholipid bilayer that encapsulates the cytoplasm and contains various components of the parental cells, including proteins, DNA, RNA, and variety of other small molecules. There are differences in the composition of exosomes from different cell sources, but they have evolutionarily conserved protein components, including integrins, transmembrane proteins, and membrane transport and fusion proteins, which are able to reflect their biological origin to some extent [26-29]. Argosomes are exosome-like vesicles containing morphogens that form a concentration gradient in the tissue, and communicate cell position by participating in signal transduction during the development of multicellular organisms. They are released from the basolateral membrane of discoid cells of drosophila, which promotes development through proliferation of epithelial cells and can participate in the direct transfer of substances between donor and recipient cells [23]. Apoptotic blebs are developed by an increase in hydrostatic pressure after cell contraction. The breakup of cell cytoskeleton results in outward bulging of the cytoplasmic membrane, which upon separation forms apoptotic blebs. Apoptotic blebs are packed with cellular organelles and chromatin to form the basis of fragmentary membrane-clad apoptotic bodies. Microvesicles are EVs between 100 nm and 1000 nm in size, formed by the regulated release of outward bulging of the plasma membrane. Currently, the best characterized EVs are exosomes and microvesicles. Table 1 summarizes the differences between exomeres, exosomes, microvesicles, argosomes, apoptotic blebs and apoptotic bodies [23-25, 30, 31]. The term exosomes is often used to refer to a mixed population of small EVs (sEV) without further proof of their intracellular origin, however, published data cannot be used to determine their precise specificity [32]. Therefore, we choose here to use the generic term EV.
|
|
Table 1 Main differences between extracellular vesicles. |
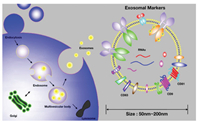
In recent years, researchers have been able to study different types of cell-derived EVs and extracted EVs from different body fluids, including plasma, serum, urine, and so on. EVs biosynthesis (Fig. 2) is a rigorous process regulated by a variety of signaling molecules [33]. Initially, receptors on the cell surface are activated, multivesicular bodies (MVBs) fuse with plasma membrane, and the vesicles are released extracellularly. EVs have become an enchanting research target in recent years due to their unique biophysical and biological characteristics. For example, they are small size in diameter, much smaller than cells (10-30 μm), but larger than proteins, so they can form a highly heterogeneous constituency. Interestingly, these properties also make it difficult to define and isolate EVs. In particular, traditional analytical tools are trammeled to efficiently and effectively detect EVs with greater clinical utility.
|
Download:
|
| Fig. 2. Illustration of exosome biogenesis and their biomarkers. Copied with permission [33]. Copyright 2019, Wiley Publishing Group. | |
As mentioned earlier, the biomarkers in exosomes are mainly proteins and nucleic acids. Proteins are mainly concentrated in the detection of membrane proteins, and nucleic acids are mainly concentrated in the detection of microRNAs. Therefore, we have classified the markers according to the detection target.
3.1. Nucleic acidsThe transport of nucleic acids inside the exosomes can play an important role in functioning of a multicellular organism [34]. Initial analyses of nucleic acids from isolated exosomes identified microRNAs (miRs) and mRNAs as the major components of exosomal cargo [35]. Exosomal miRNAs have been under most attention among exosomal nucleic acids as cancer diagnosis biomarkers due to their stability against RNase-dependent degradation [36, 37].
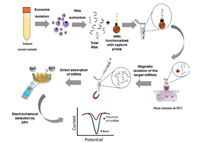
miRNAs are a small family of non-coding RNA molecules (approximately 22 nucleotides) that are an important component of EVs. miRNAs play a key regulatory role in post-transcriptional gene expression in the pathogenesis of many chronic diseases, including cancer. miRNAs in EVs are involved in tumor progression by regulating tumor microenvironment, angiogenesis, immune escape, and metastasis [38, 39]. Boriachek and coworkers reported a simple, non-amplified electrochemical method for detection of cancer-derived exosomal miRNAs in human serum samples (Fig. 3) [40]. Firstly, they captured the target miRNA by hybridization with biotinylated complimentary probes attached to commercially available streptavidin coated magnetic beads. The captured miRNA species were heat released from the hybrid and their level was subsequently monitored by the direct adsorption onto SPE-Au via RNA-gold affinity interaction, followed by differential pulse voltammetry (DPV) response in the presence of [Fe(CN)6]4-/3- redox system. Finally, the level of adsorbed miRNAs was detected by electrochemical method. The detection limit was as low as 1.0 pmol/L and the relative standard deviation (%RSD) was < 5.5%. This method showed excellent detection efficiency.
|
Download:
|
| Fig. 3. Schematic representation of the assay for detection of exosomal miRNA-21 in cancer serum samples. Copied with permission [40]. Copyright 2018, Royal Society of Chemistry. | |
In order to avoid the drift of related signals caused by environmental influences in electrochemical tests, Luo and his collaborators studied a "Y"-like structure mediated by a locked nucleic acid (LNA)-assisted strand displacement reaction (LSDR). A proportional electrochemical DNA biosensor can detect exosomal miRNA-21 (miR-21) (Fig. 4) [41]. The LNA-assisted strand displacement reaction on the "Y"-shaped structure is activated in the presence of miR-21, and the "Y"-shaped structure is then transformed into a hairpin structure, with methylene blue (MB) signal turned on and ferrocene (Fc) signal turned off. The electrochemical detection of exosomal miR-21 is achieved by changing the current ratio of MB to Fc, and the detection limit (LOD) can reach 2.3 fmol/L [41].
|
Download:
|
| Fig. 4. Schematic illustration of ratiometric electrochemical biosensor for exosomal miR-21 detection. Copied with permission [41]. Copyright 2020, Elsevier Publishing Group. | |
Exosome membrane surface proteins can reflect the characteristics and state of the cells from which they are derived and have advantage of being directly detectable without lysis. It is therefore important to use this property to develop a detection method for tumor surface specific proteins. Different methodologies for extracellular vesicles detection by tumor surface specific proteins were summarized in Table 2 [16-18, 42-54].
|
|
Table 2 Comparison of different methodologies for extracellular vesicles detection. |
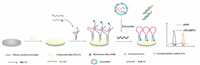
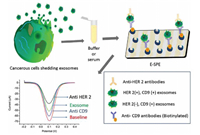
Antibodies are specific proteins that are synthesized and secreted by plasma cells after humans or animals are stimulated by antigenic substances (such as bacteria or their toxins, viruses). Because of their specificity and affinity for the target, antibodies are considered the first choice of molecular recognition units, which has also made them an excellent probe in the development of biosensors [55]. Various electrochemical assays have been developed over the past several years for the detection of EVs. Yadav suggested a streptavidin-modified screen-printed electrode to achieve highly sensitive detection of disease-specific exosomes (Fig. 5) [18]. First, four functionalized transmembrane biomarkers (CD9) were used to capture exosomes with avidin-modified screen-printed electrodes, followed by use of cancer-specific antibodies, human epidermal growth factor receptor 2 (HER-2) antibodies, to detect tumor-specific exosomes in captured liposomes, allowing quantitative detection of HER2-positive breast cancer cells. This method showed excellent specificity for HER-2(+) BT-474 cell-derived exosomes, with detection limits as low as 4.7×105 exosomes/mL, and relative standard deviation < 4.9% (n = 3). Screen-printed electrodes are inexpensive, simple and sensitive system for miniaturization and real-time analysis of exosomes. This simple and inexpensive electrochemical method has the potential to be used for future clinical bioassays.
|
Download:
|
| Fig. 5. Schematic representation of the sandwich assay for detection of disease-specific exosomes. The exosomes were isolated from the cell culture media and spiked in buffer or serum. Biotinylated CD9 antibodies were then immobilized on extra avidin-modified screen-printed carbon electrodes by using biotin avidin coupling chemistry. The subsequent addition of exosomes (spiked in buffer or serum) on the electrode surface was captured by surface-bound CD9 antibodies. The disease-specific HER-2(+) exosomes were finally detected by the HER-2 antibody. Copied with permission [18]. Copyright 2016, Wiley Publishing Group. | |
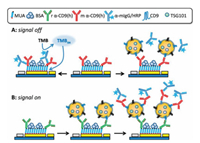
Doldan and co-workers developed an electrochemical sandwich immunosensor based on surface marker-mediated signal amplification for determination of exosomes (Fig. 6) [47]. The rabbit anti-human CD9 antibody was immobilized on a gold electrode, and then the surface protein for the captured exosomes was detected by a horseradish peroxidase (HRP)-labeled detection antibody. The generated electrical signal by the treatment with tetramethyl benzidine (TMB) was estimated for quantitative detection of the exosomes. The immune sandwich sensor has detection sensitivity as low as 200 exosomes/mL, and works well even at sample volumes as low as 1.5 mL. The linear range spans almost four orders of magnitude and demonstrates the ability to detect exosomes in real samples (diluted serum). The abovementioned biosensor can be used in future miniaturization and integrated processing of semi-automatic equipment.
|
Download:
|
| Fig. 6. "Signal-Off" and "Signal-On" Strategies. Copied with permission [47]. Copyright 2016, ACS Publishing Group. | |
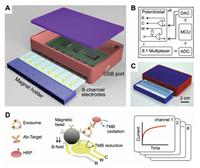
Integrated approach can also be used in the detection of EVs. For example, Jeong and co-workers acquired a portable integrated magnetic-electrochemical exosomes (iMEX) biosensor based on multiple exosomal proteins detection (Fig. 7) [16]. The new iMEX biosensor uses a multi-channel design to simultaneously detect eight specimens in 1 h, requiring only 10 μL of plasma per specimen. Exosomes were first captured from the patient samples by immunomagnetic methods and then analyzed by electrochemical reactions. This method for combining magnetic enrichment with enzymatic amplification enables a high throughput measurement of ex vivo exosomes with highly improved sensitivity. The detection limit for the iMEX biosensor was 3×104 exosomes, about 100 times higher than the ELISA.
|
Download:
|
| Fig. 7. Integrated magnetic-electrochemical exosome (iMEX) platform. (A) Sensor schematic. The sensor can simultaneously measure signals from eight electrodes. Small cylindrical magnets are located below the electrodes to concentrate immunomagnetically captured exosomes. (B) Circuit diagram. The sensor system has eight potentiostats, an 8-to-1 multiplexer, an analog-to-digital converter (ADC), a digital to-analog converter (DAC), and a micro-controller unit (MCU). Each potentiostat has three electrodes: reference (R), counter (C), and working (W). (C) A packaged device. The device has a small form factor (9×6×2 cm3). (D) Schematic of iMEX assay. Exosomes are captured on magnetic beads directly in plasma and labeled with HRP enzyme for their electrochemical detection. The magnetic beads were coated with antibodies against CD63, an enriched surface marker in exosomes. The working (W) and counter (C) electrodes were made of gold (Au), and the reference electrode (R) was made of silver/silver chloride (Ag/AgCl). Eight channels are simultaneously monitored for high-throughput analysis. HRP, horseradish peroxidase; TMB, 3, 3', 5, 5'-tetramethylbenzidine. Copied with permission [16]. Copyright 2016, ACS Publishing Group. | |
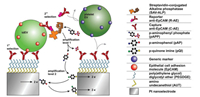
In order to improve the sensitivity of the biosensor, Mathew and co-workers reported an electrochemical detection method that combines sandwich immunoassay and enzyme-linked assays, with a combination of redox cycles on nano-finger electrodes to detect prostate tumor-derived extracellular vesicles (tdEVs) (Fig. 8) [48]. The combination of enzymatic amplification with a redox cycle between the nanoscale interdigital electrodes, confer higher detection sensitivity to the system. In addition, high specificity was achieved by two independent selections in sandwich immunoassays. The detection limits for this assay was as low as 5 tdEVs/μL, with linear response range distributed over 6 orders of magnitude (10-106 tdEVs/μL), exhibiting an excellent linear range with naturally occurring exosomes in the blood of the cancer patients. Moreover, there was a large overlap in the concentration range, and the assay was performed in a lab-on-a-chip format, allowing analysis of a small number of samples in the (low) microliter range, providing a new opportunity for future detection of clinically relevant concentrations of exosomes even from fingertip blood. This assay has the potential to be developed in the future as a portable, instant care sensing device that can be used for a wide range of disease screening.
|
Download:
|
| Fig. 8. Schematic illustration of tdEVs sensing using a sandwich immunoassay and redox cycling on nIDEs resulting in two-level selectivity and two-level amplification. tdEVs are captured using C-AE tethered to electrodes (first level of selectivity). The binding of R-AE to the tdEVs completes the antibody-antigen-antibody sandwich (second-level selectivity), after which the enzyme ALP is introduced using a biotin-SAV interaction. ALP provides an enzymatic amplification of pAPP to pAP by substrate cleavage (first-level amplification), which was followed by an electrochemical signal amplification via oxidation of pAP to pQI and subsequent redox cycling thereof between the nIDE electrodes (second level amplification). Copied with permission [48]. Copyright 2019, ACS Publishing Group. | |
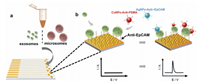
Aptamers are a type of oligonucleotide single-stranded oligonucleotides that are obtained from in vitro screening by exponential enrichment of ligand evolution technology (SELEX), and can bind to target molecules with high affinity and specificity. Compared with traditional antibodies, aptamers have the advantages of small size, stable chemical properties, and high costeffectiveness as recognition elements in biosensors. More importantly, the aptamer provides significant flexibility and convenience in structural design, which makes the new biosensor exhibit high sensitivity and selectivity [56]. Therefore, aptamers are also widely used in electrochemical biosensors. Previous study reported a sensor chip containing eleven individual round gold electrodes that enable multiple readouts and multiple analyses of exosomes using electrooxidation of nanoparticles (Fig. 9) [52]. Initially, the surface of the electrode was modified with a thiolated anti-EpCAM aptamer, to allow efficient capture of the exosomes. Then, using copper (CuNPs) and silver (AgNPs) metal nanoparticles as probes, the thiol-conjugated anti-EpCAM aptamer and the anti-PSMA aptamer were modified, and the obtained metal nanoparticles (MNPs)-aptamer were affixed. The compound was then incubated with exosomes captured by the chip, and the expression levels of EpCAM and PSMA on the exosomes were compared. This method for detecting and characterizing exosomes by identifying and labeling specific protein markers expressed on exosomes by direct electrooxidation of labeled MNPs has the advantage of being simple, rapid, cost-effective, and requires only a small amount of sample (25 μL). In addition, the method is versatile and can be used to detect exosomes from different sources by altering the capture agent on the electrode, and the identifier conjugated to the MNP. This electrochemical detection method can be used for early diagnosis of cancer.
|
Download:
|
| Fig. 9. Schematic representation of the two-step isolation and analysis of exosomes and microsomes. a) capture step where vesicles are immobilized on aptamer-modified sensors, b) electrochemical detection of the captured exosomes/microsomes with Cu and Ag nanoparticles. Copied with permission [52]. Copyright 2019, Wiley Publishing Group. | |
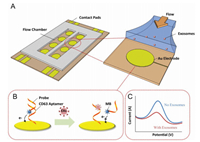
In order to establish a simpler sensing method, Zhou and coworkers reported an aptamer biosensor based on the electrochemical method using transmembrane protein CD63 to detect exosome from liver cancer cells (Fig. 10) [53]. In their method, an aptamer specific for the exosome transmembrane protein CD63 was first immobilized on the surface of the gold electrode, and then the probe chain, previously labeled with the redox moiety was then hybridized to the adaptation, anchored to the electrode surface on the body molecule. The antisense nucleic acid strand labeled with methylene blue was then paired with the aptamer strand, and the exosomes wereadded tothe biosensor. At this time, the aptamer was bound to the CD63 protein on the surface of the exosomes, thereby detection of the released exosomes was achieved by using a probe labeled with methylene blue and changing the redox signal. The aptamer biosensor has a detection limit of 1×106 exosmes/μL, which is 100 times lower than the standard immunoassay ELISA, and extends linearly to 1×108 particles/mL. This method can be easily read without labeling or washing, and has higher sensitivity than the conventional immunoassay methods. This method can be used in the future to monitor the kinetics of exosomes released from cancer cells or damaged cells.
|
Download:
|
| Fig. 10. Schematic illustrationof aptamer-based exosome detection.(A) Thedevice is composedofan Au electrodearray patternedon a glasssurface, along with a PDMSlayer, which defines the flowchamber. Aptamers specific for CD63 were immobilized onto the Au electrodes prior to use. (B and C) MB-labeled probing strands then hybridize with the aptamers anchored on the surface and emit an electrochemical signal (blue curve in Fig. C). Exosomes interact with DNA duplexes via CD63 proteins, displacing the antisense strand and decreasing the electrochemical signal (C). The change in redox signal is proportional to the concentration of exosomes in solution. Copied with permission [53]. Copyright 2016, Elsevier Publishing Group. | |
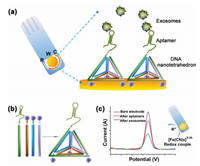
For sensitive and efficient quantification of tumor-derived exosomes, Wang and co-workers invented a nanotetrahedron (NTH)-assisted aptamer biosensor for direct capture and detection of liver-derived exosomes, by combining the nanostructures of DNA with aptamer technology and portable electrochemical devices (Fig. 11) [50]. The aptamer LZH8, also known as cell viable cells, was produced by modified in vitro selection (called cell-LIVE) that contains an extended nucleotide and four regular nucleotides. In contrast to the experimental control, cancerous hepatocytes (Hu1545) showed superior binding selectivity on target hepatoma cells (HepG2). Moreover, the NTH structures, composed of selfassembled four single-stranded sequences, were used to improve specificity and capture efficiency, and can be combined with electrochemically detected aptamer sensors. The aptamerfunctionalized electrode was then incubated with isolated exosomal suspension, at which point the immobilized aptamer can be directly and specifically captured by the exosomes. The detection limit for the LZH8-dependent aptamer sensor was 3.96×105 exosomes/mL, while the detection limit for the NTHdependent aptamer sensor was 2.09×104 exosomes/mL. This method provides a proof of concept for sensitive and efficient quantification of tumor-derived exosomes and also lays the foundation for quantitative study of exosomes in more complex body fluids.
|
Download:
|
| Fig. 11. Schematic illustration of NTH-assisted electrochemical aptasensor. (a) Aptamer-containing NTHs were immobilized via three thiol groups onto the gold electrodes for direct capture of exosomes in suspension. R: reference electrodearea; W: working electrode area, with a diameter of 4 mm; C: counter electrode area. (b) Facile self-assembly of DNA nanotetrahedra from four single-stranded DNA sequences were mixed in equal concentrations, followed by heating at 95 ℃ for 2 min and "snap-cooling" at 4 ℃ for 1 min. (c) Redox signal changes after aptamer immobilization and after incubation with exosomes. One drop of 5 mmol/L K3[Fe(CN)6]/100 mmol/L KCl can cover the entire electrode area for signal generation and amplification. Copied with permission [50]. Copyright 2017, ACS Publishing Group. | |
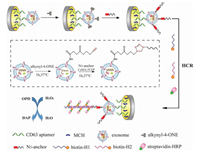
Recently, An and co-workers used a signal-amplified DNA hybridization chain reaction (HCR) to establish an electrochemical aptamer biosensor based on click chemistry, to achieve ultrasensitive detection of tumor exosomes (Fig. 12) [17]. The CD63 aptamer was first immobilized on a glassy carbon electrode, to capture the exosomes, and then the functionalized lipid electrophile 4-oxo-2-nonene alkyne (alkynyl-4) was reacted by the reaction between an amino group and an aldehyde group, which conjugates the molecule to the exosomes. The alkynyl-4-ONE showed high reactivity toward proteins, and was used to modify exosomes derived from MCF-7 cells, followed by conjugation of an azide-labeled DNA probe as an anchor through a copper(I)- catalyzed click chemistry reaction. The azide-labeled DNA probe was attached as an anchor to the exosomes. By adding auxiliary DNA, the HCR forms a long self-assembling DNA linker that can be used for signal amplification, while a large number of linked horseradish peroxidase (HRP) molecules can catalyze o-phenylenediamine (OPD) and H2O2 reaction. Finally, the concentration of exosomes was quantified by monitoring the electrochemical reduction current of 2, 3-diaminophenazine (DAP). Under the optimum conditions, the LOD for this method was 96 particles/mL, and the detection range was 1.12×102 particles/μL to 1.12×108 particles/μL. In addition, it was resistant to interference from serum proteins, and the mentioned characteristics for this method show that it also has great potential for quantitative analysis of tumor exosomes in clinical diagnosis.
|
Download:
|
| Fig. 12. Schematic illustration of the proposed aptasensor for exosome detection based on click chemistry and HCR for signal amplification. Copied with permission [17]. Copyright 2019, Elsevier Publishing Group. | |
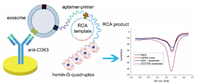
Taking into account the specificity of the aptamers and advantages of rolling circle amplification (RCA), our group has lately reported an immunochemical aptasensor for sensitive detection of gastric cancer-derived exosomes, by combining heme/G-quadruplex system with RCA (Fig. 13) [54]. First, gastric cancer cells or cancer overexpressed protein aptamers were screened to obtain gastric cancer exosomes specific aptamer, which was used as a detection probe. Next, anti-CD63 bound to the exosomal surface marker was used as a capture probe to capture different kinds of exosomes. Among these exosomes, only gastric cancer exosomes can trigger RCA to generate a large number of G quadruplets. The unit was generated and the resulting product was incubated with heme to form a heme-G-quadruplex structure, which in turn catalyzed the H2O2 system to produce an electrochemical signal. The aptasensor exhibited high selectivity and sensitivity to gastric cancer exosomes and can be used to detect plasma of gastric cancer patients. The detection limit obtained by the experiment was 9.54×102 exosomes/mL, and the linear response range was 4.8×103 exosomes/mL to 4.8×106 exosomes/mL. Since the expression of MUC1 is a useful marker, this high selectivity and sensitivity aptasensor can be used for the diagnosis and prognosis of gastric cancer, especially predicting poor prognostic factors associated with lymphatic metastasis in early gastric cancer.
|
Download:
|
| Fig. 13. Illustration of the label-free electrochemical aptasensor for highly sensitive detection of exosomes. Copied with permission [54]. Copyright 2019, Wiley Publishing Group. | |
In this review, we discussed the electrochemical biosensors' advantages, such as enhanced capture, reduced assay time and matrix effect, as well as simplified and inexpensive device manufacturing for detection of EVs. The electrochemical biosensors have great advantage in EVs detection field and can have potential implications in cancer screening, prediction of patient prognosis and therapeutic applications. But it still faces the challenge of surface functionalization, sample matrix effects and reproducibility problems. Therefore, the interfacial engineering investigation, preconcentration of exosomes, and stable electrode and nanoparticles, will be the potential breakthrough strategy [57].
It is well known that DNA, lipids, and peptides can also be used as biomarkers for the detection of EVs [58-61]. However, there have been no reports on EVs electrochemical detection, but their detection based on other biosensor methods have been reported [62-66]. By using these reviewed advanced technologies, research in related fields such as tumor source, tumor stage [67], and tumor specificity [68] can successfully be performed.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (Nos. 61971216 and 61527806), National Key Research and Development Program of China (No. 2017YFA0205301), the Jiangsu Province Medical Talent (No. ZDRCA2016065), the Key Research and Development Project of Jiangsu Province (No. BE2019603), the Health Care for Cadres Research Fund of Jiangsu Province (No. BJ16003), High-level Health Talent Project of Jiangsu Province (No. LGY2019001).
| [1] |
P.D. Stahl, G. Raposo, Physiology Bethesda (Bethesda) 34 (2019) 169-177. |
| [2] |
D.R. Santiago-Dieppa, D.D. Gonda, V.J. Cheung, et al., Intracranial Gliomas Part III-Innovative Treatment Modalities, Karger Publishers, Basel, 2018, pp. 172-179.
|
| [3] |
M. Colombo, G. Raposo, C. Théry, Annu. Rev. Cell. Dev. Bi. 30 (2014) 255-289. DOI:10.1146/annurev-cellbio-101512-122326 |
| [4] |
J. Kowal, M. Tkach, C. Théry, Curr. Opin. Cell Biol. 29 (2014) 116-125. DOI:10.1016/j.ceb.2014.05.004 |
| [5] |
E. Cocucci, J. Meldolesi, Trends Cell Biol. 25 (2015) 364-372. DOI:10.1016/j.tcb.2015.01.004 |
| [6] |
J. Zhang, S. Li, L. Li, et al., Genom. Proteom. Bioinf. 13 (2015) 17-24. DOI:10.1016/j.gpb.2015.02.001 |
| [7] |
S. Keerthikumar, D. Chisanga, D. Ariyaratne, et al., J. Mol. Biol. 428 (2016) 688-692. DOI:10.1016/j.jmb.2015.09.019 |
| [8] |
C. Lässer, M. Eldh, J. Lötvall, JoVE (2012) e3037.
|
| [9] |
A. Michael, S.D. Bajracharya, P.S. Yuen, et al., Oral Dis. 16 (2010) 34-38. DOI:10.1111/j.1601-0825.2009.01604.x |
| [10] |
X. Zhang, X. Yuan, H. Shi, et al., J. Hematol. Oncol. 8 (2015) 83.
|
| [11] |
X. Lv, Z. Geng, Y. Su, et al., Langmuir 35 (2019) 9816-9824. DOI:10.1021/acs.langmuir.9b01237 |
| [12] |
H. Shao, J. Chung, K. Lee, et al., Nat. Commun. 6 (2015) 6999.
|
| [13] |
J.R. Chevillet, Q. Kang, I.K. Ruf, et al., Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 14888-14893. DOI:10.1073/pnas.1408301111 |
| [14] |
T. An, S. Qin, Y. Xu, et al., J. Extracell. Vesicles 4 (2015) 27522.
|
| [15] |
S.A. Melo, L.B. Luecke, C. Kahlert, et al., Nature 523 (2015) 177-182. DOI:10.1038/nature14581 |
| [16] |
S. Jeong, J. Park, D. Pathania, et al., ACS Nano 10 (2016) 1802-1809. DOI:10.1021/acsnano.5b07584 |
| [17] |
Y. An, T. Jin, Y. Zhu, F. Zhang, P. He, Biosens. Bioelectron. 142 (2019) 111503.
|
| [18] |
S. Yadav, K. Boriachek, M.N. Islam, et al., Chem. Electro. Chem. 4 (2017) 967-971. |
| [19] |
M. Lin, P. Song, G. Zhou, et al., Nat. Protoc. 11 (2016) 1244-1263. DOI:10.1038/nprot.2016.071 |
| [20] |
K.M. Koo, L.G. Carrascosa, M.J. Shiddiky, M. Trau, Analy. Chem. 88 (2016) 2000-2005. DOI:10.1021/acs.analchem.5b04795 |
| [21] |
M.H. Haque, V. Gopalan, S. Yadav, et al., Biosens. Bioelectron. 87 (2017) 615-621. DOI:10.1016/j.bios.2016.09.016 |
| [22] |
N.J. Ronkainen, H.B. Halsall, W.R. Heineman, Chem. Soc. Rev. 39 (2010) 1747-1763. DOI:10.1039/b714449k |
| [23] |
F.T. Borges, L. Reis, N. Schor, Braz. J. Med. Biol. Res. 46 (2013) 824-830. DOI:10.1590/1414-431X20132964 |
| [24] |
H. Zhang, D. Freitas, H.S. Kim, et al., Nat. Cell Biol. 20 (2018) 332-343. DOI:10.1038/s41556-018-0040-4 |
| [25] |
Q. Zhang, J.N. Higginbotham, D.K. Jeppesen, et al., Cell Rep. 27 (2019) 940-954. DOI:10.1016/j.celrep.2019.01.009 |
| [26] |
Z. Wei, A.O. Batagov, S. Schinelli, et al., Nat. Commun. 8 (2017) 1145. DOI:10.1038/s41467-017-01196-x |
| [27] |
C.S. Hong, S. Funk, L. Muller, M. Boyiadzis, T.L. Whiteside, J. Extracell, Vesicles 5 (2016) 29289. DOI:10.3402/jev.v5.29289 |
| [28] |
W. Li, C. Li, T. Zhou, et al., Mol. Cancer 16 (2017) 145. DOI:10.1186/s12943-017-0706-8 |
| [29] |
A. Li, T. Zhang, M. Zheng, Y. Liu, Z. Chen, Oncol. 10 (2017) 175. DOI:10.1186/s13045-017-0542-8 |
| [30] |
J.C. Akers, D. Gonda, R. Kim, B.S. Carter, C.C. Chen, J. Neurooncol. 113 (2013) 1-11. DOI:10.1007/s11060-013-1084-8 |
| [31] |
S. Mathivanan, H. Ji, R.J. Simpson, J. Proteomics 73 (2010) 1907-1920. DOI:10.1016/j.jprot.2010.06.006 |
| [32] |
M. Tkach, C. Théry, Cell 164 (2016) 1226-1232. DOI:10.1016/j.cell.2016.01.043 |
| [33] |
K. Kalishwaralal, W.Y. Kwon, K.S. Park, Biotechnol. J. 14 (2019) 1800430. 10.1186/s13036-019-0160-9
|
| [34] |
O. Gusachenko, M. Zenkova, V. Vlassov, Biochemistry (Moscow) 78 (2013) 1-7. DOI:10.1134/S000629791301001X |
| [35] |
H. Valadi, K. Ekström, A. Bossios, et al., Nat. Cell Biol. 9 (2007) 654-659. DOI:10.1038/ncb1596 |
| [36] |
P.S. Mitchell, R.K. Parkin, E.M. Kroh, et al., Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 10513-10518. DOI:10.1073/pnas.0804549105 |
| [37] |
Y.H. Soung, S. Ford, V. Zhang, J. Chung, Cancers 9 (2017) 8. DOI:10.3390/cancers9010008 |
| [38] |
D. Bhagirath, T.L. Yang, N. Bucay, et al., Cancer Res. 78 (2018) 1833-1844. DOI:10.1158/0008-5472.CAN-17-2069 |
| [39] |
A. Thind, C. Wilson, J. Extracell, Vesicles 5 (2016) 31292. 10.3402/jev.v5.31292
|
| [40] |
K. Boriachek, M. Umer, M.N. Islam, et al., Analyst 143 (2018) 1662-1669. DOI:10.1039/C7AN01843F |
| [41] |
L. Luo, L. Wang, L. Zeng, et al., Talanta 207 (2020)120298.
|
| [42] |
R. Vaidyanathan, M. Naghibosadat, S. Rauf, et al., Analy. Chem. 86 (2014) 11125-11132. DOI:10.1021/ac502082b |
| [43] |
H. Im, H. Shao, Y.I. Park, et al., Nat. Biotechnol. 32 (2014) 490-495. DOI:10.1038/nbt.2886 |
| [44] |
M. Oliveira-Rodríguez, S. López-Cobo, H.T. Reyburn, et al., J. Extracell. Vesicles 5 (2016) 31803.
|
| [45] |
Z. Zhao, Y. Yang, Y. Zeng, M. He, Lab Chip 16 (2016) 489-496. DOI:10.1039/C5LC01117E |
| [46] |
S. Zong, L. Wang, C. Chen, et al., Anal. Methods 8 (2016) 5001-5008. DOI:10.1039/C6AY00406G |
| [47] |
X. Dolda'n, P. Fagu'ndez, A. Cayota, J. Laíz, J.P. Tosar, Analy. Chem. 88 (2016) 10466-10473. DOI:10.1021/acs.analchem.6b02421 |
| [48] |
D.G. Mathew, P. Beekman, S.G. Lemay, et al., Nano Lett. 20 (2020) 820-828. DOI:10.1021/acs.nanolett.9b02741 |
| [49] |
Y. Jiang, M. Shi, Y. Liu, et al., Angew. Chem. Int. Ed. 56 (2017) 11916-11920. DOI:10.1002/anie.201703807 |
| [50] |
S. Wang, L. Zhang, S. Wan, et al., ACS Nano 11 (2017) 3943-3949. DOI:10.1021/acsnano.7b00373 |
| [51] |
X. Chen, J. Lan, Y. Liu, et al., Biosens. Bioelectron. 102 (2018) 582-588. DOI:10.1016/j.bios.2017.12.012 |
| [52] |
Y.G. Zhou, R.M. Mohamadi, M. Poudineh, et al., Small 12 (2016) 727-732. DOI:10.1002/smll.201502365 |
| [53] |
Q. Zhou, A. Rahimian, K. Son, et al., Methods 97 (2016) 88-93. DOI:10.1016/j.ymeth.2015.10.012 |
| [54] |
R. Huang, L. He, Y. Xia, et al., Small 15 (2019) 1900735.
|
| [55] |
D. Saerens, L. Huang, K. Bonroy, S. Muyldermans, Sensors 8 (2008) 4669-4686. DOI:10.3390/s8084669 |
| [56] |
S. Song, L. Wang, J. Li, C. Fan, J. Zhao, TrAC-Trend. Anal. Chem. 27 (2008) 108-117. DOI:10.1016/j.trac.2007.12.004 |
| [57] |
N. Cheng, D. Du, X. Wang, et al., Trends Biotechnol. 37 (2019) 1236-1254. DOI:10.1016/j.tibtech.2019.04.008 |
| [58] |
F. He, H. Liu, X. Guo, B.C. Yin, B.C. Ye, Anal. Chem. 89 (2017) 12968-12975. DOI:10.1021/acs.analchem.7b03919 |
| [59] |
B.K. Thakur, H. Zhang, A. Becker, et al., Cell Res. 24 (2014) 766. 10.1038/cr.2014.44
|
| [60] |
Y. Wan, G. Cheng, X. Liu, et al., Nat. Biomed. Eng. 1 (2017) 0058. 10.1038/s41551-017-0058
|
| [61] |
H. Shao, H. Im, C.M. Castro, et al., Chem. Rev. 118 (2018) 1917-1950. DOI:10.1021/acs.chemrev.7b00534 |
| [62] |
R. Duraichelvan, B. Srinivas, S. Badilescu, et al., ECS Trans. 75 (2016) 11-17. |
| [63] |
C. Lee, R. Carney, K. Lam, J.W. Chan, J. Raman Spectrosc. 48 (2017) 1771-1776. DOI:10.1002/jrs.5234 |
| [64] |
Q. Tian, C. He, G. Liu, et al., Anal. Chem. 90 (2018) 6556-6562. DOI:10.1021/acs.analchem.8b00189 |
| [65] |
T. Skotland, K. Sandvig, A. Llorente, Prog. Lipid Res. 66 (2017) 30-41. DOI:10.1016/j.plipres.2017.03.001 |
| [66] |
H. Bu, D. He, X. He, K. Wang, ChemBioChem 20 (2019) 451-461. DOI:10.1002/cbic.201800470 |
| [67] |
Y. Yoshioka, Y. Konishi, N. Kosaka, et al., J. Extracell. Vesicles 2 (2013) 20424. 10.3402/jev.v2i0.20424
|
| [68] |
A. Hoshino, B. Costa-Silva, T.L. Shen, et al., Nature 527 (2015) 329.
|
 2020, Vol. 31
2020, Vol. 31