b School of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
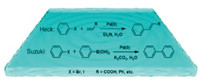
In Suzuki and Heck coupling reactions of aryl halides (Scheme 1), palladium nanoparticles have been investigated as catalysts for the coupling reactions in lieu of Pd salts or various forms of Pd complexes [1-7]. The expensive Pd salts or complexes in homogeneous catalysis often suffer from complicated separation and recovery problems in the reaction media or metal contamination of the products, which have hitherto prevented their wide industrial applications. The thermodynamically unstable Pd nanoparticles protected by a polymer can be used as active catalysts for coupling phenylboronic acids with aryl iodides [8, 9]. The reactions were shown to have initial rates dependent linearly on the concentration of the Pd catalyst. This phenomenon was attributed to the surface reaction mechanism occurring on the Pd nanoparticles [10]. However, it was difficult to reuse the catalyst because the Pd nanoparticles were dispersed in water and precipitated over time during the reactions, resulting in significant loss of catalytic activity [11].
|
Download:
|
| Scheme 1. Suzuki and Heck coupling reactions catalyzed by Pd nanoparticles using water as solvent. | |
Efforts were made to improve the effect of stabilizers on the catalytic activity and stability of Pd nanoparticles in the Suzuki coupling reactions [12-14]. Among the several types of stabilizers, e.g., polystyrene-b-poly(sodium acrylate) and hydroxyl-terminated poly(amido-amine) (PAMAM) dendrimers, Pd nanoparticles stabilized with the dendrimers were the least active because the Pd particles were so tightly encapsulated that adsorption of reaction substrates was hindered. In addition, significant homocoupling of the phenylboronic acid occurred, resulting in lower activity. The PAMAM-stabilized Pd nanoparticles were active when using aqueous ethanol or DMF as solvent [15]. Still, formation of Pd black precipitates in the reactions using stabilized colloidal particles has been an issue [2, 12, 16]. Cyclodextrin-capped Pd nanoparticles used for the Suzuki coupling [17] prevented the substrates from accessing to ca. 50% of the Pd surface, and caused the solubility mismatch between the hydrophobic reaction substrates and the hydrophilic CD-Pd particles. Microgel-stabilized Pd nanoclusters were more active than the Pd(Ⅱ) precursors in the Suzuki couplings [18], presumably because the latter need to be reduced in situ before they could enter the catalytic cycle. Again, Pd precipitation is a problem, which lowers the activity and reusability in these systems.
A polymer matrix with metal nanoparticles (so called hybrid or composite) may exhibit synergistic effect in both useful functionality and mechanical integrity in variable reaction conditions [19-25]. It is known that a heterogeneous Pd catalyst is required because homogeneous Pd catalysts are not reusable and need strong coordination ligands (e.g., toxic phosphine) or chelating agents to stabilize the catalytically active Pd(0) species and complicated purification steps to remove the residual Pd and ligand after reactions are completed. Thus, heterogeneous Pd catalysts, including the immobilization or stabilization of Pd nanoparticles on ionic liquids [16], functionalized polymers [26], graphene oxide and its derivatives [8], and inorganic substrates such as silica, zeolites [9], have been developed. However, reaction conditions for large-scale Suzuki applications need to be more environmentally benign with a lower reaction temperature and water solvent [10].
Our previous study showed that Heck coupling reactions were efficiently catalyzed by Pd nanoparticles immobilized on polymer hydrogels and recycled over 20 times, demonstrating more sustainability than any other polymer-stabilized Pd catalysts reported in the literature [27]. Although the identity of the true catalytic species with supported Pd nanoparticles or complex precatalysts is still in dispute, recent studies have demonstrated that supported Pd catalysts can serve as a reservoir of leached Pd being active for C-C coupling reactions [28-33]. Recognizing that the Pd metals undergo redox reactions during the reactions and leaching, we hereby use hydrogel-supported palladium catalysts for use in C-C bond forming reactions. In this study, Pd nanoparticles immobilized in crosslinked polymers and interpenetrating polymer network (IPN) hydrogels are employed to catalyze Suzuki and Heck reactions and achieve an optimal balance between recyclability (like a heterogeneous catalysis) and activity (like a homogeneous catalysis) in aqueous media. This approach maximizes the water solvation ability of the hydrogels and restricts the leaching of Pd inside the hydrogels, or reduces their free leaching (Scheme S1 in Supporting information). Thus, the Pd metals are oxidized and reduced inside the hydrogels and IPN networks. The approach shown here indicates a dramatic low dosage of the Pd required for the coupling reactions.
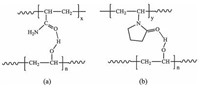
Three sets of hydrogels are used to disperse the Pd nanoparticles. The first consists of crosslinked poly(vinyl alcohol) (cPVA) alone; in the second and third sets, crosslinked poly (acrylamide) (PAM) or poly(N-vinyl pyrrolidone) (PVP) networks are introduced into the PVA networks, respectively, to form interpenetrating networks, and simultaneously, to maintain hydrogen bonds between the side chains, in addition to the H-bonds between gel networks and water molecules trapped in the network. As shown in Scheme 2, new interpolymer complexes can be formed between the side chains of the PVA and PAM (or PVP) polymer networks. More importantly, the presence of an additional polymer network firmly entraps the Pd nanoparticles and soluble (unstable) smaller Pd clusters/species possibly generated in the coupling reactions in the networks.
|
Download:
|
| Scheme 2. Formation of interpolymer complexes: (a) between vinyl alcohol and acrylamide repeating units in the PVA-PAM IPN; (b) between vinyl alcohol and N-vinylpyrrolidone repeating units in the PVA-PVP IPN. | |
The Pd nanoparticles embedded in the crosslinked PVA polymers (Pd@cPVA) were synthesized by reducing aqueous Pd2+ in PVA in the first step. Formation of the Pd nanoparticles with the change in the color of the mixture to black was confirmed by TEM. Figs. 1a–c show typical TEM images of the Pd nanoparticles in three types of polymer networks. The Pd particles are highly dispersed in the polymer matrices, with a size (in average diameter ± standard deviation) of 25.04±10.23 nm, 25.25±11.81 nm, and 37.38±13.64 nm in cPVA, IPN (PVA-PAM) and IPN (PVA-PVP) polymers, respectively. The Pd particles have their size distributions illustrated in Figs. 1d–f. Comparison with Fig. 1d, Figs 1e and f show that addition of the PAM chain network has essentially retained the average size of the Pd particles in the cPVA, while introduction of the PVP chain has moderately increased the average size of the Pd particles. In all three cases, the Pd particles are highly dispersed with nearly unimodal distributions in the polymers.
The cPVA and IPN dispersion phases are essentially invisible in the TEM images in Figs. 1a–c. The polymers prepared here act as nano-porous matrices in the hybrid architecture. Preparation of Pd nanoparticles with average diameters of about 4 nm was also made; however, such particles were found not stable enough to show good catalytic recyclability. Therefore, it is important to have particle sizes large enough to be bound in the networks for reusable performance, rather than merely to obtain smaller nanoparticles. On the other hand, good dispersion of the Pd particles in the polymers shown in Figs. 1a–c is important for reproducibility of the catalytic performance. If the hydrogels and IPN structures were prepared in the absence of the Pd metals, and impregnated with the Pd(Ⅱ) salts which were later reduced by ethanol or NaBH4 reagent through soaking, the Pd nanoparticles were found to be much larger (around 100 nm) and dispersed nonuniformly in the hydrogels, resulting in poor reproducibility of the catalytic activities.
H-Bonding of the polymer network with water molecules leads to swelling of the hydrogels, allowing the reactants to have easy access to the Pd metals. The pores in the hydrogels also create hydrophilic nano-environments in the network structure.
Several types of Suzuki coupling reactions have been tested between bromobenzene (or iodobenzene) and phenylboronic acid. As shown in Table 1, good yields of biphenyl derivatives from the cross-coupling reactions are obtained when the reactions are run at 100 ℃ for 4 h in a mixed medium of DMAc/water =1/20 (v/v), with an aid of a phase transfer agent TBAB (2.5 μmol). It was found that the turnover frequency (TOF) was as high as 1800 h-1 for the coupling of bromobenzene with phenylboronic acid (entry 1). The TOF values are higher than previously reported Pd nanoparticles or nano-rods for Suzuki reaction [15, 28, 29], and at least two orders of magnitude higher than the value of Pd nanoparticle/PVP/CMK-3 catalysts reported previously [30]. Phenyl bromides or iodides also reacted efficiently with phenylboronic acid substituted with an electron-donating methoxy- group or with an electron-withdrawing acetyl group, producing the biaryl products with good yields (entries 3-6).
|
|
Table 1 Results of Suzuki reactions catalyzed by Pd@cPVA and Pd@IPN.a |
The asymmetric reaction products in entries 3-7 (Table 1) show that homo-coupling between the phenylboronic acid derivatives is absent. Besides, when the bromobenzene or iodobenzene was removed in the reaction, no products were found. Although homocoupling of the phenylboronic acid during Suzuki reactions was recently reported in the literature [12, 30], such a side reaction is not competitive in the present system.

|
Download:
|
| Fig. 1. TEM of Pd nanoparticles in (a) Pd@cPVA; (b) Pd@IPN (PVA-PAM); (c) Pd@IPN (PVA-PVP) hydrogel catalyst. Scale bar = 500 nm. Size distributions of Pd nanoparticles in (d) Pd@cPVA; (e) Pd@IPN (PVA-PAM); (f) Pd@IPN (PVA-PVP) hydrogel catalyst. | |
TBAB may promote the migration of the base from the aqueous phase to the organic reactants to neutralize the protonated Pd(Ⅱ) complexes produced in the catalytic redox cycle, facilitating the regeneration of the active zerovalent Pd catalyst and carrying the resulting salt into the aqueous phase. When the TBAB was removed, the yield was lowered to 35% in the co-solvent with the same ratio of DMAc/water 1/20 (Table 2, entry 4).
|
|
Table 2 Effect of co-solvent constituent and TBAB on the Suzuki reaction.a |
The biphenyl production was also affected by the specific ratio of the organic solvent/water used (Table 2). The coupling reaction can occur with a good yield when the DMAc/water ratio is increased to 1/1 (v/v), without TBAB in the reaction feed (Table 2, entry 5). Therefore, the constituents of the co-solvents play an important role in the Suzuki reactions. An appropriate ratio of water/organic solvent may stabilize reaction intermediate species on the Pd nanoparticle surfaces and promote the reaction.
Reaction kinetics of Suzuki reaction between iodobenzene and phenylboronic by IPN catalyst are performed (Figs. S5–S7 in Supporting information). Products increase with the reaction time and temperature. The catalytic reaction is a rate-limiting step, since the diffusion step is affected by the intermolecular interaction (H-bonding) between reactants and IPN networks that does not involve scission or formation of covalent bonds. It is easy to overcome the H-bonding due to its low bonding energy (21-29 kJ/mol). However, the activation energy of the catalytic reaction is 38.94 kJ/mol including adsorption process on the catalyst surface and covalent bond scission and formation, which is much higher than that of H-bonding.
The recyclability of the Pd@IPN and Pd@cPVA catalysts was tested by repeatedly carrying out Suzuki coupling reactions at 100 ℃ in DMAc:H2O = 1:1 co-solvent without TBAB. After each cycle, the catalyst was filtered off, thoroughly washed with water and ethanol, and dried in the air and re-used in the Suzuki reaction. The Pd@IPN catalyst showed excellent activity for the coupling reaction of iodobenzene with phenylboronic acid after seven consecutive runs, retaining a product yield of 77% in the 7th run. In comparison, the Pd@cPVA catalyst showed good activity for the coupling reaction of iodobenzene with phenylboronic acid for at least five consecutive runs, with a yield of 80% in the 5th run. These results are better than those obtained with other heterogeneous catalysts in the literature. For instance, Pd nanoparticles immobilized by conjugated polymers are recyclable up to three runs, reducing the yield to 76% at the end of the third cycle [31]. Pd salts on hydrotalcite only retained 20% of their activity for Suzuki reaction after the third cycle of re-use, and only about 10% after the fourth catalytic cycle [32, 33]. Carbon-supported Pd nanoparticles could maintain 71% of their catalytic activity after five cycles of the Suzuki reaction [34]. Polystyrene–poly(ethylene glycol) resincaptured Pd nanoparticles were recyclable up to six times [20]. Longer re-usable performance of the Pd@IPN catalysts in this study indicates even higher TOF values and better cost-effectiveness for industrial applications if the whole life cycle of each batch of the catalysts is calculated before deactivation.
Several factors are believed to be associated with the recyclable performance. First, formation of IPN chain structures may favor the binding of the polymers with Pd nanoparticles, when compared with the simple cPVA hydrogel. Second, formation of interpolymer complexes shown in Scheme 2 may further entrap the Pd nanoclusters/species in the networks, leading to the improved recyclability of the Pd@IPN versus that of the Pd@cPVA. Any palladium species dissolved from the particles during the course of the reaction [35, 36] may be instantly re-bound and re-deposited inside the hydrogel networks, therefore, ensuring the Pd metals are tightly entrapped without precipitation in the solvent. As for the decreased yield after several cycles of reaction, we proposed that there is the Ostwald ripening effect, which involves the migration of Pd metals from smaller particles and re-deposition to larger particle surfaces during the catalytic reaction [27]. Larger particles may have lower activities. Synthesis of more narrowly distributed nanoparticles may alleviate this problem.
A series of Heck coupling reactions in water was also studied for derivatives of phenyl iodide and acrylic acid. The results listed in Table 3 show that the products were (E)-cinnamic acid derivatives. The yields were dependent on the nature of the substituents on the phenyl ring. Heck coupling reactions are often carried out in polar aprotic organic solvents, such as DMF or DMSO, with a Pd2+ salt, in the presence of a strong ligand, such as a toxic ligand phosphine, which stabilizes the Pd(0) atoms as complexes and prevents the Pd(0) from fast precipitation in the solutions. The Pd@IPN system here does not require such ligands, organic solvent or a phase transfer reagent for the Heck reactions. The turnover frequencies (TOFs) calculated from the isolated yields, the amount of the Pd used, and reaction time, are about 50 times larger than those of the recently reported polyelectrolyte complex stabilized Pd nanoparticles [37].
|
|
Table 3 Results of Heck reactions catalyzed by Pd@IPN.a |
In summary, Pd nanoparticles could be highly dispersed in crosslinked PVA and IPN hydrogels and utilized in Suzuki and Heck cross-coupling reactions. Their high catalytic activity for the coupling reactions (high turnover frequencies) with hydrophobic substrates were demonstrated. Furthermore, the hydrogels could be recovered from the aqueous solutions by simple filtration, like a heterogeneous reaction. The catalysts were successfully reused with good catalytic activity in the repeated recycling studies. The IPN support shows better recyclability than the crosslinked PVA alone. The combined benefits for the coupling reactions and the improved recyclability indicate that the Pd/hydrogel hybrids are cost-effective sustainable catalyst reactors [38, 39]. Moreover, the Pd metal loadings in the polymer hydrogels in this study are much lower than those in other polymeric or organic frameworks or mesoporous carbons reported in the literatures [31, 40-42]. This type of hybrid catalysts is valuable because of its mild reaction conditions.
AcknowledgmentsThis work was supported by Natural Science Foundation of Fujian Province of China (No. 2018J06003), Natural Science Foundation of Zhejiang Province (Nos. Y4090504 and LY12E03001), Department of Science and Technology of Zhejiang Province (No. 2012C24003) and the National Natural Science Foundation of China (Nos. 21973061 and 21674063).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.006.
| [1] |
A.J. Reay, I.J. Fairlamb, Chem. Commun. 51 (2015) 16289-16307. DOI:10.1039/C5CC06980G |
| [2] |
A. Biffis, P. Centomo, A. Del Zotto, M. Zecca, Chem. Rev. 118 (2018) 2249-2295. DOI:10.1021/acs.chemrev.7b00443 |
| [3] |
A. Balanta, C. Godard, C. Claver, Chem. Soc. Rev. 40 (2011) 4973-4985. DOI:10.1039/c1cs15195a |
| [4] |
L. Liu, A. Corma, Chem. Rev. 118 (2018) 4981-5079. DOI:10.1021/acs.chemrev.7b00776 |
| [5] |
A. Molnar, Chem. Rev. 111 (2011) 2251-2320. DOI:10.1021/cr100355b |
| [6] |
S.J. Guo, J. Bai, H.O. Liang, C.P. Li, Chin. Chem. Lett. 27 (2016) 459-463. DOI:10.1016/j.cclet.2015.12.029 |
| [7] |
L. Zhang, W.H. Dong, N.Z. Shang, et al., Chin. Chem. Lett. 27 (2016) 149-154. DOI:10.1016/j.cclet.2015.08.007 |
| [8] |
Y. Li, X.M. Hong, D.M. Collard, M.A. El-Sayed, Org. Lett. 2 (2000) 2385-2388. DOI:10.1021/ol0061687 |
| [9] |
R. Narayanan, M.A. El-Sayed, J. Am. Chem. Soc. 125 (2003) 8340-8347. DOI:10.1021/ja035044x |
| [10] |
Y. Li, E. Boone, M.A. El-Sayed, Langmuir 18 (2002) 4921-4925. DOI:10.1021/la011469q |
| [11] |
R. Narayanan, M.A. El-Sayed, J. Phys. Chem. B 108 (2004) 8572-8580. DOI:10.1021/jp037169u |
| [12] |
Y. Li, M.A. El-Sayed, J. Phys. Chem. B 105 (2001) 8938-8943. DOI:10.1021/jp010904m |
| [13] |
F. Durap, A. Baysal, D. Elma, M. Aydemir, Ö. Ok, Z. Baysal, Synth. React. Inorg. Met. 46 (2016) 1402-1409. DOI:10.1080/15533174.2015.1136954 |
| [14] |
D. Kale, G. Rashinkar, A. Kumbhar, R. Salunkhe, React. Funct. Polym. 116 (2017) 9-16. |
| [15] |
M. Pittelkow, K. Moth-Poulsen, U. Boas, J.B. Christensen, Langmuir 19 (2003) 7682-7684. DOI:10.1021/la0348822 |
| [16] |
M. Pérez-Lorenzo, J. Phys. Chem. Lett. 3 (2012) 167-174. DOI:10.1021/jz2013984 |
| [17] |
L. Strimbu, J. Liu, A.E. Kaifer, Langmuir 19 (2003) 483-485. DOI:10.1021/la026550n |
| [18] |
A. Biffis, E. Sperotto, Langmuir 19 (2003) 9548-9550. DOI:10.1021/la0350709 |
| [19] |
D. Kundu, A.K. Patra, J. Sakamoto, H. Uyama, React.Funct. Polym. 79 (2014) 8-13. DOI:10.1016/j.reactfunctpolym.2014.03.002 |
| [20] |
J.K. Cho, R. Najman, T.W. Dean, et al., J. Am. Chem. Soc. 128 (2006) 6276-6277. DOI:10.1021/ja057480k |
| [21] |
S. Ogasawara, S. Kato, J. Am. Chem. Soc. 132 (2010) 4608-4613. DOI:10.1021/ja9062053 |
| [22] |
P.M. Uberman, L.A. Pérez, G.I. Lacconi, S.E. Martín, J. Mol. Catal. A:Chem. 363 (2012) 245-253. |
| [23] |
M.S.A.S. Shah, D. Guin, S.V. Manorama, Mater. Chem. Phys. 124 (2010) 664-669. DOI:10.1016/j.matchemphys.2010.07.031 |
| [24] |
P. Centomo, M. Zecca, M. Kralik, et al., J. Mol. Catal. A:Chem. 300 (2009) 48-58. DOI:10.1016/j.molcata.2008.10.039 |
| [25] |
Y. Zhang, J. Yang, X. Zhang, F. Bian, W. Yu, React.Funct.Polym. 72 (2012) 233-241. DOI:10.1016/j.reactfunctpolym.2012.02.010 |
| [26] |
Y. Wang, C. Lu, G. Yang, Z. Chen, J. Nie, React. Funct. Polym. 110 (2017) 38-46. DOI:10.1016/j.reactfunctpolym.2016.12.003 |
| [27] |
K. Zhan, H. You, W. Liu, et al., React. Funct. Polym. 71 (2011) 756-765. DOI:10.1016/j.reactfunctpolym.2011.04.007 |
| [28] |
N.T. Phan, M. Van Der Sluys, C.W. Jones, Adv. Synth. Catal. 348 (2006) 609-679. DOI:10.1002/adsc.200505473 |
| [29] |
A.K. Diallo, C. Ornelas, L. Salmon, J. Ruiz Aranzaes, D. Astruc, Angew. Chem. Int. Ed. 46 (2007) 8644-8648. DOI:10.1002/anie.200703067 |
| [30] |
L. Joucla, G. Cusati, C. Pinel, L. Djakovitch, Appl. Catal. A:Gen. 360 (2009) 145-153. DOI:10.1016/j.apcata.2009.03.016 |
| [31] |
S. J.-Chen, A.N. Vasiliev, A.P. Panarello, J.G. Khinast, Appl. Catal. A:Gen. 325 (2007) 76-86. DOI:10.1016/j.apcata.2007.03.010 |
| [32] |
S.P. Andrews, A.F. Stepan, H. Tanaka, S.V. Ley, M.D. Smith, Adv. Synth. Catal. 347 (2005) 647-654. DOI:10.1002/adsc.200404331 |
| [33] |
J.M. Richardson, C.W. Jones, J. Catal. 251 (2007) 80-93. DOI:10.1016/j.jcat.2007.07.005 |
| [34] |
H. Y.-Chen, H. H.-Hung, M.H. Huang, J. Am. Chem. Soc. 131 (2009) 9114-9121. DOI:10.1021/ja903305d |
| [35] |
Y. Ma, X. Ma, Q. Wang, J. Zhou, Catal. Sci. Technol. 2 (2012) 1879-1885. DOI:10.1039/c2cy00001f |
| [36] |
L.A. Adrio, B.N. Nguyen, G. Guilera, A.G. Livingston, K.K.M. Hii, Catal. Sci. Technol. 2 (2012) 316-323. DOI:10.1039/C1CY00241D |
| [37] |
R.U. Islam, M.J. Witcomb, M.S. Scurrell, et al., Catal.Sci.Technol. 1 (2011) 308-315. DOI:10.1039/c0cy00071j |
| [38] |
J.R. Ruiz, C. Jiménez-Sanchidrián, M. Mora, Tetrahedron 62 (2006) 2922-2926. |
| [39] |
M. Mora, C. Jiménez-Sanchidrián, J.R. Ruiz, J. Colloid Interface Sci. 302 (2006) 568-575. DOI:10.1016/j.jcis.2006.06.058 |
| [40] |
R. Narayanan, M.A. El-Sayed, J. Catal. 234 (2005) 348-355. DOI:10.1016/j.jcat.2005.06.024 |
| [41] |
S.S. Soomro, F.L. Ansari, K. Chatziapostolou, K. Köhler, J.Catal. 273 (2010) 138-146. DOI:10.1016/j.jcat.2010.05.007 |
| [42] |
A. Ohtaka, Y. Tamaki, Y. Igawa, et al., Tetrahedron 66 (2010) 5642-5646. DOI:10.1016/j.tet.2010.05.076 |
 2020, Vol. 31
2020, Vol. 31