b Research Institute of Sun Yat-Sen University in Shenzhen, Shenzhen 518057, China
Derma is the biggest organs of the body and the first barrier avoiding from pathogens from the external environment [1, 2]. As derma is exposed to air, various injury or pathogenic factors can lead to skin trauma, which necessitates rapid repair. Skin wound healing occurs through overt overlapping phases: hemostasis, inflammation, proliferation and remodeling [3, 4]. During the inflammation phase, to protect against the invading microbes, neutrophils are catalyzed and proteases and reactive oxygen species (ROS) are released at the wound site. However, the overproduction of ROS could impede the wound healing process because it could give rise to serious damage of the cells such as fibroblasts which secretes collagen and glycosaminoglycans that are beneficial to tissue structure remodeling for wound repair.
To address, antioxidant material has been frequently utilized for wound treatment and management as its ability to reduce the negative effects of wounds by removing the generated ROS and promote wound healing [5-8]. Up to now, the most popular antioxidants applied for wound healing include phenols [9-12] and flavones [13], such as curcumin [14-16], tea polyphenol [17, 18] and so on [19, 20]. In recent years, whole grain has caught attentions as it is rich in phenolic compounds and has potentials to provide natural antioxidants. Among them, wheat grain, in which the major identified phenolic composition includes phenolic acids and flavonoids, has been studied for its antioxidant activity [21, 22]. However, as far as we know, there have been no systematical study about the application of wheat grain antioxidant activity for wound healing. One report discussed the radicals scavenging activities of whole wheat flour extract by organic solvents such as acetone, ethyl acetate, diethyl ether and so on [23], but they were almost not further explored for wound healing application which may be the residues of organic solvents.
Here, we found that the whole wheat flour aqueous solution behaved both excellent antioxidant activity and biocompatibility, demonstrating the potential application for wound healing. Based on the detection of biocompatibility, whole wheat flour solution (WWFS) was explored in vitro through MTT assay, DAPI/ FITC-phalloidin and AO/EB staining and hemolysis activity assay. What is more, WWFS could scavenge radical in vitro and intracellular ROS induced by hydrogen peroxide (H2O2) in protecting cells or skin tissues from oxidative stress. Most importantly, a full-thickness in vivo cutaneous wound model confirmed WWFS promoted collagen deposition, vessel blood and hair follicles regeneration in wound site.
To improve the biocompatibility of WWFS, a convenient and new method was used here by DMEM, avoiding potential cytotoxicity caused by residual organic solvents. The cells viability of different concentrations of WWFS was carried out by MTT assay in mouse NIH 3T3 fibroblasts (Fig. 1a). The result showed that the viability of NIH 3T3 cells all remained over 84% when the concentration of solution was generally 1 mg/mL or less (0.1, 0.2, 0.3 and 0.5 mg/mL) after incubation for 1, 3, and 5 d respectively, which was considered to be good cytocompatibility, while beyond 1 mg/mL the cell viability was below 60% on the third day. Herein, the following experiments were carried out based on 0.1, 0.2, 0.3, 0.5 and 1 mg/mL whole wheat flour solutions (WWFSs). Furthermore, cellular morphology and apoptosis were analyzed by DAPI/ FITC-phalloidin and AO/EB staining, respectively. From Fig. 1b, the morphology was not affected after incubation for 1 d when cells were exposed to the low concentration of solution. Besides, incubated 1 d, there was almost no apoptosis, and although proceeded on the second day, the apoptosis in a normal level was observed in a concentration-dependent manner (Figs. 1d and f), which was matched with the cell viability by MTT assay. Besides, the hemolysis activity of WWFSs was tested by direct contact method [24]. Fig. 1c showed hemolysis ratios for the positive control sample, negative control sample, and WWFSs groups from low to high. The hemolysis ratios of different WWFSs groups and negative control sample were very similar. The supernatant was almost colorless as the red blood cells (RBCs) were sedimented at the bottom after tubes were centrifuged, which demonstrated that WWFSs owned excellent anti-hemolytic properties. As shown in Fig. 1e, the hemolysis percentage of all WWFSs groups were about 1% which were well low compared to the maximum limit (5%), indicating that WWFSs behave excellent blood compatibility.

|
Download:
|
| Fig. 1. The biocompatibility of different concentration of whole wheat flour solutions (WWFSs) (0.1, 0.2, 0.3, 0.5 and 1 mg/mL) in vitro. (a) The cell proliferation in WWFSs detected by the MTT assay (n = 3). (b) The morphology of NIH 3T3 cells in different concentration of whole wheat flour solutions (0.1, 0.2, 0.3, 0.5 and 1 mg/mL) after 1 d incubation, analyzed by DAPI/FITC-phalloidin staining. (c) Images of RBCs after being treated with (1) water, (2) sodium chloride solution, (3) 0.1, (4) 0.2, (5) 0.3, (6) 0.5 and (7) 1 mg/mL WWFSs. (d) The apoptosis of NIH 3T3 cells treated with WWFSs after 1 d, analyzed by AO/EB staining (apoptosis: white arrows). (e) Hemolysis ratio analysis of RBCs after incubation with sodium chloride solution as negative group, different concentration of WWFSs or water as positive group (n = 3). (f) The apoptosis of NIH 3T3 cells treated with WWFSs after 2 d, analyzed by AO/EB staining (apoptosis: white arrows). | |
After that, the radical scavenging ability of WWFSs was evaluated based on antioxidant assay, hydroxyl radical scavenging assay [25]. Briefly, hydroxyl radical generated by Fenton reaction react with sodium salicylate to produce 2, 3-dihydroxybenzoic acid with the absorption peak at 510 nm, which has a positive correlation with free radicals. As shown in Fig. 2a, WWFSs could effectively scavenge free radicals in a concentration-dependent manner, which may depend on the increasing phenolic compounds. From the result, the anti-radical efficiency of 0.3 mg/mL solution was burst improved, and 0.5 mg/mL (18%) was close to 1 mg/mL (18.2%) after 30 min. Furthermore, solutions could inhibit the oxidative stress effect of NIH 3T3 cells induced by H2O2, which were reflected in cells by the fluorescent dye DCFH-DA in vitro. As shown in Fig. 2b(1), low intracellular ROS levels were produced by cells without any stimulation. In contrast, the cellular ROS level was significantly increased after pre-treated with H2O2 solution (100 μmol/L) (Fig. 2b(2)) observed through strong fluorescence, but was significantly attenuated by pretreatment with 0.1 and 0.2 mg/mL WWFSs (Fig. 2b(3, 4)). Notably, when pretreatment with the solutions that were higher than or equal to 0.3 mg/mL, there were almost no fluorescence found in the cells. The results described that WWFSs exhibited good intracellular antioxidant activity to promote the growth of NIH 3T3 cells that helped reduce inflammation and promoted tissue regeneration.

|
Download:
|
| Fig. 2. The radical scavenging activity of various concentration of whole wheat flour solutions (WWFSs). (a) Anti-radical efficiency detected by hydroxyl radical scavenging. (b) The cellular ROS detected by DCFH-DA, (1) negative control group, (2) positive control group, (3) 0.1, (4) 0.2, (5) 0.3, (6) 0.5 and (7) 1 mg/mL WWFSs groups. | |
In this study, the SD rat full thickness cutaneous wound model was built to investigate the effect of WWFS as coating on wound healing. Taken together, 0.5 mg/mL WWFS was used as coating for promoting wound healing. As shown in Fig. 3a, the WWFS could promote wound healing because the skin wound closure area of WWFS group was greater than that of the control group on day 10 and 20. The wound closure area in the WWFS group reached 83%, while the control group only achieved 71% after 10 days (Fig. 3b). On the 21st day, WWFS-treated wound completely closed, but the obvious wound was still found in the control group, demonstrating that WWFS accelerated wound healing in vivo. For histological analysis, specimens were used for preparing hematoxylin and eosin (H&E), Masson's Trichrome and Immunohistochemical staining (Fig. 3c). From H&E and Masson's staining, it was found that wounds treated with WWFS exhibited a higher degree of well-organized granulation tissue, characterized by abundant blood vessel distribution and increased collagen fibers aggregation in a Besides, there were some hair follicles exhibited in WWFS group. However, the control group not only exhibited uncomplete re-epithelialization, but also displayed few hair follicles. On the contrary, the epithelial tissue was regenerated completely and closer to normal skin in WWFS group. More skin appendages like hair follicles and blood vessels were observed in WWFS group and exhibited significant differences when compared with control group as well (Fig. 3d). Using immunohistochemistry to explore VEGF expression, representing the formation of blood vessels, showed that WWFS improved blood vessel amount as compared to the control group, which contributed to wound healing. The results of histological staining revealed that WWFS accelerated the regeneration of new skin tissues.
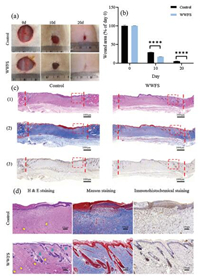
|
Download:
|
| Fig. 3. The effect in promoting wound healing of 0.5 mg/mL whole wheat flour solution (WWFS) in vivo. (a) Representative images of wounds of the control and WWFS groups in days 10 and 20. (b) Wound area for each group; (c) Representative images of wounds of the control and WWFS groups detected by (1) H&E, (2) Masson and (3) Immunohistochemical staining on 20th day (two vertical red lines: the size of the wound). (d) Magnification of Representative images (red square of Fig. 3c) of wounds detected by H&E (blood vessels: yellow arrows; hair follicles: blue arrows), Masson and Immunohistochemical staining on 20th day. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. | |
In conclusion, whole wheat flour solution group showed better tissue regeneration, compared with control group in wound closure rate, collagen deposition, re-epithelization, hair follicles and vessel blood regeneration, which may be related with its proper antioxidant property exhibited in scavenging radicals and ROS induced by reactive oxygen species. In addition, good biocompatibility of WWFSs were demonstrated with many tools, such as MTT assay, hemolysis activity assay, DAPI/FITC- phalloidin and AO/EB staining, providing the potential application of whole grain in wound healing.
AcknowledgmentsWe sincerely acknowledge the funding and generous support from financial support from National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2018ZX10301402), International Cooperation and Exchange of the National Natural Science Foundation of China (No. 51820105004), Guangdong Innovative and Entrepreneurial Research Team Program (Nos. 2013S086 and 2016ZT06S029), Science and Technology Program of Guangzhou (No. 201707010094), Science and Technology Planning Project of Shenzhen (No. JCYJ20170307141438157) and the Fundamental Research Funds for the Central Universities (No. 18lgpy58).
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.011.
| [1] |
A.L. Byrd, Y. Belkaid, J.A. Segre, Nat. Rev. Microbiol. 16 (2018) 143-155. DOI:10.1038/nrmicro.2017.157 |
| [2] |
A. Summerfield, F. Meurens, M.E. Ricklin, Mol. Immunol. 66 (2015) 14-21. DOI:10.1016/j.molimm.2014.10.023 |
| [3] |
S. Dekoninck, C. Blanpain, Nat. Cell Biol. 21 (2019) 18-24. DOI:10.1038/s41556-018-0237-6 |
| [4] |
Malone-Povolny M., S. Maloney, M Schoenfisch, Adv. Healthcare Mater. (2019) 1801210. |
| [5] |
I. Süntar, E.K. Akkol, L. Nahar, S.D. Sarker, Free. Radic. Antioxid. 2 (2012) 1-7. |
| [6] |
Gallego-Villar L., B. Pérez, M. Ugarte, L.R. Desviat, E. Richard, Biochem. Biophys. Res. Commun. 452 (2014) 457-461. DOI:10.1016/j.bbrc.2014.08.091 |
| [7] |
N. Abuid, K. Gattas-Asfura, E. Schofiled, C Stabler, Adv. Healthcare Mater. (2019) 1801493. |
| [8] |
M. Guo, S. Bi, J. Liu, et al., Chin. Chem. Lett. 28 (2017) 1889-1892. DOI:10.1016/j.cclet.2017.07.021 |
| [9] |
C.Y. Tsai, L.C. Woung, J.C. Yen, et al., Carbohydr. Polym. 135 (2016) 308-315. DOI:10.1016/j.carbpol.2015.08.098 |
| [10] |
Y. Zheng, X. You, S. Guan, et al., Adv. Funct. Mater. 29 (2019) 1808646. DOI:10.1002/adfm.201808646 |
| [11] |
Y. Song, R. Zeng, L. Hu, et al., Biomed. Pharmacother. 93 (2017) 451-461. DOI:10.1016/j.biopha.2017.06.079 |
| [12] |
E. Yadav, D. Singh, P. Yadav, A. Verma, Biomed. Pharmacother. 96 (2017) 86-97. DOI:10.1016/j.biopha.2017.09.125 |
| [13] |
Y. Tsai, C.G. Lin, W.L. Chen, et al., Agronomy-Basel 9 (2019) 27. DOI:10.3390/agronomy9010027 |
| [14] |
J. Liu, Z. Chen, J. Wang, et al., ACS Appl. Mater. Interfaces 10 (2018) 16315-16326. DOI:10.1021/acsami.8b03868 |
| [15] |
Y. Fan, J. Yi, Y. Zhang, W. Yokoyama, Food Chem. 239 (2018) 1210-1218. DOI:10.1016/j.foodchem.2017.07.075 |
| [16] |
X. Huang, X. Huang, Y. Gong, et al., Food Res. Int. 87 (2016) 1-9. DOI:10.1016/j.foodres.2016.06.009 |
| [17] |
B. Romana-Souza, T.C. Pires, A. Monte-Alto-Costa, Food Res. Int. 71 (2015) 32-40. DOI:10.1016/j.foodres.2015.02.018 |
| [18] |
H. Shahrahmani, N. Kariman, S. Jannesari, et al., Phytother. Res. 32 (2018) 522-530. DOI:10.1002/ptr.5999 |
| [19] |
H. Zhang, T. Rong, Curr. Opin. Food Sci. 8 (2016) 33-42. DOI:10.1016/j.cofs.2016.02.002 |
| [20] |
S.T. Gao, G.S. Tang, D.W. Hua, et al., J. Mater. Chem. B 7 (2019) 709-729. DOI:10.1039/C8TB02491J |
| [21] |
Y. Lu, A. Memon, P. Fuerst, et al., J. Food Compos. Anal. 60 (2017) 10-16. DOI:10.1016/j.jfca.2017.03.001 |
| [22] |
K. Masisi, T. Beta, M.H. Moghadasian, Food Chem. 196 (2016) 90-97. DOI:10.1016/j.foodchem.2015.09.021 |
| [23] |
Y. Pang, S. Ahmed, Y. Xu, et al., Food Chem. 240 (2018) 212-221. DOI:10.1016/j.foodchem.2017.07.095 |
| [24] |
Y.W. Xi, J. Ge, Y. Guo, B. Lei, P.X. Ma, ACS Nano 12 (2018) 10772-10784. DOI:10.1021/acsnano.8b01152 |
| [25] |
K. Lalhminghlui, G.C Jagetia, Future Sci. OA 4 (2018) FSO272. DOI:10.4155/fsoa-2017-0086 |
 2020, Vol. 31
2020, Vol. 31 

