b Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Tianjin 300071, China
The development of efficient methods for the synthesis and site-selective functionalization of nitrogen-containing heterocycles, particularly quinolines, is important because of their presence in drugs [1], natural products [2], and functional materials [3] and their utility as templates for asymmetric synthesis [4]. One reaction for this purpose is transition-metal-catalyzed C—H bond functionalization, which is an expedient method for synthesizing complex molecules owing to its atom and step economy and the ready availability of the starting materials [5]. However, controlling the regioselectivity for substrates with multiple reactive C—H bonds is a key challenge inherent to this approach. A common powerful strategy for achieving regioselectivity is the use of directing groups, although the need to install and remove such groups makes this strategy relatively inefficient. This inefficiency could be circumvented by choosing a directing group that need not be removed.
An ideal directing group for controlling the regioselectivity of transition-metal-catalyzed C—H bond functionalization is the quinoline N-oxide group. A number of methods have been developed for direct C2 functionalization of quinoline N-oxides, including alkylation [6], olefination [[7]], arylation [7b, 8], sulfo-nylation [9], acetoxylation [10], phosphonation [11], amination [12], and carbamoylation [13]. In contrast, only a few methods for the introduction of functional groups at the remote C8 position of quinoline N-oxides have been reported [14]. For example, Chang et al. [14a] and Sawamura et al. [14g] reported direct C8 arylation and borylation, respectively, which use the nitrogen atom in the quinoline moiety as a directing group. The method reported by Chang et al. requires a dirhodium NHC (N-heterocyclic carbine) complex.
More recently, Larionov's group developed a protocol for PdII-catalyzed C8 arylation with ArI, but high temperature and a large excess of acetic acid are required (Scheme 1a) [14j]. Chang's group achieved an IrIII-catalyzed C8 arylation reaction by using aryl diazonium salts as arylation reagents (Scheme 1b) [14i], but only eight examples were reported. Samanta's group reported RhIII-catalyzed C8 arylation involving diazonaphthalen-2(1H)-ones as coupling partners [14w]. Finally, Li et al. showed that a RhIII-catalyzed C8 arylation/methylation sequence can be achieved with organotrifluoroborates, but the yields are only moderate (Scheme 1c) [14t].

|
Download:
|
| Scheme 1. Transition-metal-catalyzed C8 arylation of quinoline N-oxides. | |
After carefully studying the literature, we reasoned that quinoline N-oxides could also be arylated by using commercially available arylboronic species as arylation reagents, and herein we report the first protocol for RhIII-catalyzed remote (C8) arylation of quinoline N-oxides with arylboronic acids (Scheme 1d). This protocol offers an efficient, regioselective route to the desired 8-arylated products from a broad range of substrates.
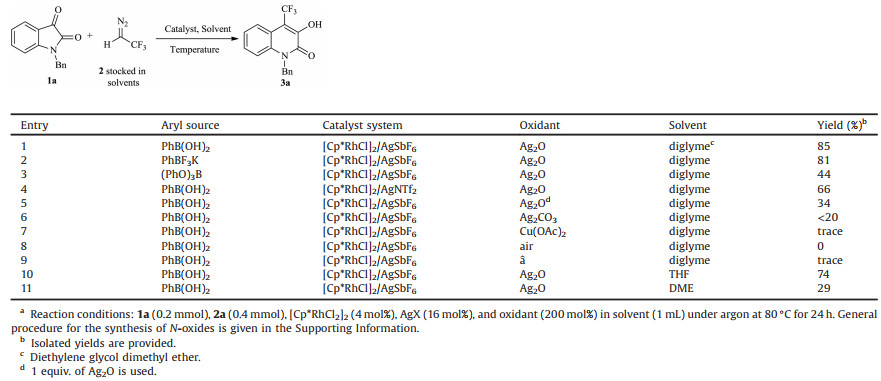
Our initial investigation was performed with quinoline N-oxide (1a), phenylboronic acid (2a) (2 equiv.), [Cp*RhCl2]2 (4 mol%), AgSbF6 (16 mol%), and Ag2O (200 mol%) under argon in diethylene glycol dimethyl ether at 80 ℃ (Table 1). To our delight, desired product 3aa was obtained in an isolated yield of 85%(entry 1), so we used these conditions to screen a series of aryl coupling partners. The use of PhBF3K and (PhO)3B decreased the yield of 3aa to 81% and 44%, respectively (entries 2 and 3); that is, PhB(OH)2 was the best of the tested aryl sources. Replacing AgSbF6 with AgNTf2 gave 3aa in only 66% yield (entry 4). Subsequently, several oxidants were screened (Ag2CO3, Cu(OAc)2, and air), but none of them were effective (entries 5–7). No product was observed in the absence of oxidant (entry 8). Tests of other ether solvents (THF and DME, entries 9 and 10) showed that diethylene glycol dimethyl ether was the best solvent. Taken together, these results indicate that the optimal conditions for the RhIII-catalyzed C8 arylation were as follows: 4 mol% [Cp*RhCl2]2, 16 mol% AgSbF6, and 2 equiv. of Ag2O in diethylene glycol dimethyl ether under argon at 80 ℃ for 24 h.
|
|
Table 1 Optimization of reaction conditions.a |
With the optimal reaction conditions in hand, we then turned our attention to an exploration of the reactions of a wide range of N-heteroaromatic oxides with phenylboronic acid (2a) to afford C8 arylated N-heteroaryl products (Scheme 2). Reactions with quinoline N-oxides bearing various electron-donating or-with-drawing substituents (F, Cl, Br, Me, OMe, and CO2Me) at C6 afforded the desired C8 arylated products (3ba–3ga) in good to excellent yields (68%–91%). Interestingly, 6-Cl-substituted substrate 1c gave the best yield under the standard reaction conditions. When C2 of the quinoline N-oxide was substituted with a Me (2h) or Ph (2i) group, the corresponding products were obtained in 82% and 88% yields, respectively. Substrates with a substituent at C3, C4, C5, or C7 also gave the corresponding products (3j–3m), although the yield of 3ma was relatively low, perhaps due to steric hindrance. Disubstituted quinoline N-oxides 1n–1r were tolerated well and provided the desired products in good yields. It is worth noting that the presence of a Cl or a Br substituent in the product opens the possibility of further functionalization by means of transition-metal-catalyzed cross-coupling reactions. Quinoxaline mono-oxide regioselectively provided reduced C8 arylation product 3sa in acceptable yield (51%)[15]. The polycondensed heteroarene phenanthridine N-oxide also gave a good yield of the corresponding arylated product (3ta).

|
Download:
|
| Scheme 2. Scope of the reaction with respect to the N-heteroaromatic oxide. Isolated yields are given. | |
Next, the scope of the reaction with respect to the arylboronic acid was examined (Scheme 3). The reaction of quinoline N-oxide (1a) with a variety of phenylboronic acids 2 substituted with an electron-donating or -withdrawing group at C4 (4-F, 4-Cl, 4-Br, 4-Me, 4-tBu, 4-Ph, 4-CN, 4-CF3 and 4-CO2Me) afforded C8 arylated products 3ab–3aj with excellent regioselectivity in moderate to good yields. A phenylboronic acid with a 2- or 3-Cl substituent also affords the corresponding products (3ak and 3al) in similar moderate yields. The substrates with an electron-donating substituent at C3 gave a higher yield than did with an electron-withdrawing substituent (compare 3am with 3an and 3ao). Disubstituted (2p, 2q) and trisubstituted (2r) phenylboronic acids were also tolerated well and provided the desired products in acceptable yields. Notably, 2-naphthaleneboronic acid was also compatible with the reaction conditions, affording corresponding product 3as in 80% yield. The thiophenyl boranic acid was also provided the desired product 3at in 49% yield, no products were obtained when other heteroaryl boronic acides such as furanyl and pyridyl boronic acides were used.

|
Download:
|
| Scheme 3. Scope of the reaction with respect to the arylboronic acid. Isolated yields are given. | |
To demonstrate the synthetic utility of this protocol, we carried out the reaction of quinolone N-oxide (1a) and phenylboronic acid (2a) on a gram scale at a catalyst loading of only 2 mol% and obtained a 73 isolated yield of 3aa (Scheme 4a). In addition, the N-oxide of quinoxyfen could be derivatized at the 8-position to give 3ua in 70% yield (Scheme 4b).

|
Download:
|
| Scheme 4. Gram-scale reaction and late-stage functionalization of the N-oxide of quinoxyfen. | |
To further demonstrate the synthetic utility of the C8 arylation products, we conducted a series of transformations of 3aa (Scheme 5). The N-oxide moiety of 3aa could be readily substituted at the C2 with a MeO group (4, 85%) or a Cl atom (5, 80%). In addition, 2-quinolone 6 was obtained in 90% yield by means of a trifluoroacetic-anhydride-mediated rearrangement [16] of 3aa. Finally, 3aa was easily reduced by PCl3 [17] to afford the corresponding 8-phenylquinoline (7) in 72% yield.

|
Download:
|
| Scheme 5. Transformations of a C8 arylation product. | |
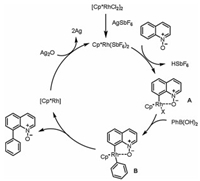
A plausible reaction mechanism for the coupling of 1a with 2a is shown in Scheme 6 [14, 18]. First, the [Cp*RhCl2]2 dimer is converted to a cationic species via a ligand switch. Coordination between Cp*RhIII and the O atom of the N-oxide moiety is followed by the formation of the key five-membered-ring rhodacyclic complex (A) via electrophilic cleavage of the C8–H bond. Next, transmetalation of A with phenylboronic acid 2 affords intermediate B; electron-donating substituents may accelerate this step. Finally, a C—C reductive elimination reaction of B furnishes desired coupling product 3aa and a RhI species. AgI-mediated reoxidation of RhI regenerates RhIII, completing the catalytic cycle.
|
Download:
|
| Scheme 6. Plausible reaction mechanism. | |
In summary, we have developed a protocol for RhIII-catalyzed direct C8 arylation of quinoline N-oxides with arylboronic acids as coupling partners. Quinoline N-oxides reacted smoothly with arylboronic acids with high regioselectivity for the C8 position of the quinoline N-oxides. The reaction could be carried out on a gram scale and was used for late-state functionalization of a fungicide. We expect that this arylation protocol will serve as a powerful tool for the synthesis of arylated compounds for medicinal chemistry and materials science applications.
Declaration of competing interestThe authors declare no competing financial interest.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China (Nos. 21977056, 21732002, 21672117, 21602117, 21772104, 31760527) and the Tianjin Natural Science Foundation (No. 16JCZDJC32400) for generous financial support for our programs.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.028.
| [1] |
(a) Z. Wu, Z. Zhen, J.H. Jiang, G.L. Shen, R.Q. Yu, J. Am. Chem. Soc. 131 (2009) 12325-12332; (b) W.A. Denny, B.F. Cain, G.J. Atwell, et al., J. Med. Chem. 25 (1982) 276-315; (c) A.B. Burgin, O.T. Magnusson, J. Singh, et al., Curr. Med. Chem. 18 (2011) 1488-1508. |
| [2] |
(a) J.vanGeer, J.A.J.Hanraads, R.A.Lupton, etal., J.Sci.Commun.163 (2010)51-59; (b) G. Höfle, B. Kunze, J. Nat. Prod. 71 (2008) 1843-1849; (c) J.P. Michael, Nat. Prod. Rep. 24 (2007) 223-246; (d) Q.A. Khan, J. Lu, S.M. Hecht, J. Nat. Prod. 72 (2009) 438-442. |
| [3] |
(a) J.I. Kim, I.S. Shin, H. Kim, J.K. Lee, J. Am. Chem. Soc. 127 (2005) 1614-1615; (b) G. Yang, Y. Si, Z. Su, Org. Biomol. Chem. 10 (2012) 8418-8425. |
| [4] |
(a) M. Nakajima, M. Saito, M. Shiro, S.I. Hashimoto, J. Am. Chem. Soc.120 (1998) 6419-6420; (b) S.E. Denmark, Y. Fan, J. Am. Chem. Soc. 124 (2002) 4233-4235; (c) M.S. Taylor, E.N. Jacobsen, PNAS 101 (2004) 5368-5373; (d) C. Verrier, P. Melchiorre, Chem. Sci. 6 (2015) 4242-4246; (e) M. Moliterno, R. Cari, A.A. Antenucci, et al., Angew. Chem. Int. Ed. 55 (2016) 6525-6529. |
| [5] |
(a) A.E. Shilov, G.B. Shul0pin, Chem. Rev. 97 (1997) 2879-2932; (b) R.G. Bergman, Nature 446 (2007) 391-393; (c) O. Daugulis, H.Q. Do, D. Shabashov, Acc. Chem. Res. 42 (2009) 1074-1086; (d) D.A. Colby, R.G. Bergman, J.A. Ellman, Chem. Rev. 110 (2010) 624-655; (e) L. Ackermann, Chem. Rev. 111 (2011) 1315-1345; (f) K.M. Engle, T.S. Mei, M. Wasa, J.Q. Yu, Acc. Chem. Res. 45 (2011) 788-802; (g) B.J. Li, Z.J. Shi, Chem. Soc. Rev. 41 (2012) 5588-5598; (h) J. Yamaguchi, A.D. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 51 (2012) 8960-9009; (i) G.Y. Song, F. Wang, X.W. Li, Chem. Soc. Rev. 41 (2012) 3651-3678; (j) J. Wencel-Delord, F. Glorius, Nat. Chem. 5 (2013) 369-375; (k) J.R. Hummel, J.A. Boerth, J.A. Ellman, Chem. Rev. 117 (2017) 9163-9227; (l) J. Kim, K. Shin, S. Jin, D. Kim, S. Chang, J. Am. Chem. Soc.141 (2019) 4137-4146. |
| [6] |
(a) J. Ryu, S.H. Cho, S. Chang, Angew. Chem. Int. Ed. 51 (2012) 3677-3681; (b) B. Yao, R.J. Song, Y. Liu, et al., Adv. Synth. Catal. 354 (2012) 1890-1896; (c) Z.Y. Wu, C. Pi, X.L. Cui, J. Bai, Y.J. Wu, Adv. Synth. Catal. 355 (2013) 1971-1976; (d) B. Xiao, Z.J. Liu, L. Liu, Y. Fu, J. Am. Chem. Soc. 135 (2013) 616-619; (e) O.V. Larionov, D. Stephens, A. Mfuh, G. Chavez, Org. Lett.16 (2014) 864-869; (f) A.K. Jha, N. Jain, Chem. Commun. 52 (2016) 1831-1834; (g) S.J. Yu, H.L. Sang, S.Z. Ge, Angew. Chem. Int. Ed. 56 (2017) 15896-15900. |
| [7] |
(a) K.S.Kanyiva, Y.Nakao, T.Hiyama, Angew.Chem.Int.Ed.46 (2007)8872-8874; (b) S.H. Cho, S.J. Hwang, S. Chang, J. Am. Chem. Soc. 130 (2008) 9254-9256; (c) J.L. Wu, X.L. Cui, L.M. Chen, G.J. Jiang, Y.J. Wu, J. Am. Chem. Soc. 131 (2009) 13888-13889; (d) F. Roudesly, L.F. Veiros, J. Oble, G. Poli, Org. Lett. 20 (2018) 2346-2350. |
| [8] |
(a) L.C. Campeau, D.R. Stuart, J.P. Leclerc, et al., J. Am. Chem. Soc. 131 (2009) 3291-3306; (b) D.B. Zhao, W.H. Wang, F. Yang, et al., Angew. Chem. Int. Ed. 48 (2009) 3296-3300; (c) P.H. Xi, F. Yang, S. Qin, et al., J. Am. Chem. Soc. 132 (2010) 1822-1824; (d) H. Wang, Y. Pei, J. Bai, et al., RSC Adv. 4 (2014) 26244-26246; (e) X.P. Chen, X.L. Cui, F.F. Yang, Y.J. Wu, Org. Lett. 17 (2015) 1445-1448. |
| [9] |
(a) Z.Y. Wu, H.Y. Song, X.L. Cui, et al., Org. Lett. 15 (2013) 1270-1273; (b) B.N. Du, P. Qian, Y. Wang, et al., Org. Lett. 18 (2016) 4144-4147. |
| [10] |
X. Chen, C.W. Zhu, X.L. Cui, Y.J. Wu, Chem. Commun. 49 (2013) 6900-6902. DOI:10.1039/c3cc43947j |
| [11] |
H. Wang, X.L. Cui, Y. Pei, et al., Chem. Commun. 50 (2014) 14409-14411. DOI:10.1039/C4CC07060G |
| [12] |
(a) G. Li, C.Q. Jia, K. Sun, Org. Lett. 15 (2013) 5198-5201; (b) C.W. Zhu, M.L. Yi, D.H. Wei, et al., Org. Lett. 16 (2014) 1840-1843; (c) W.L. Xie, J.H. Yoon, S. Chang, J. Am. Chem. Soc. 138 (2016) 12605-12614. |
| [13] |
B. Yao, C.L. Deng, Y. Liu, et al., Chem. Commun. 51 (2015) 4097-4100. DOI:10.1039/C4CC10140E |
| [14] |
(a) J. Kwak, M. Kim, S. Chang, J. Am. Chem. Soc. 133 (2011) 3780-3783; (b) H.Hwang, J.Kim, J.Jeong, S.Chang, J.Am.Chem.Soc.136 (2014)10770-10776; (c) X.T. Zhang, Z.S. Qi, X.W. Li, Angew. Chem. Int. Ed. 53 (2014) 10794-10798; (d) J. Jeong, P. Patel, H. Hwang, S. Chang, Org. Lett. 16 (2014) 4598-4601; (e) T. Shibata, Y. Matsuo, Adv. Synth. Catal. 356 (2014) 1516-1520; (f) U. Sharma, Y. Park, S. Chang, J. Org. Chem. 79 (2014) 9899-9906; (g) S. Konishi, S. Kawamorita, T. Iwai, et al., Chem. Asian J. 9 (2014) 434-438; (h) Y. Park, K.T. Park, J.G. Kim, S. Chang, J. Am. Chem. Soc.137 (2015) 4534-4542; (i) K. Shin, S.W. Park, S. Chang, J. Am. Chem. Soc. 137 (2015) 8584-8592; (j) D.E. Stephens, J. Lakey-Beitia, A.C. Atesin, et al., ACS Catal. 5 (2015) 167-175; (k) D.E. Stephens, J. Lakey-Beitia, G. Chavez, et al., Chem. Commun. 51 (2015) 9507-9510; (l) D. Gwon, H. Hwang, H.K. Kim, S.R. Marder, S. Chang, Chem. Eur. J. 21 (2015) 17200-17204; (m)R.Sharma, R.Kumar, I.Kumar, U.Sharma, Eur.J.Org.Chem.2015 (2015)7519-7528; (n) X.H. Hu, X.F. Yang, T.P. Loh, ACS Catal. 6 (2016) 5930-5934; (o) N. Barsu, M. Sen, J.R. Premkumar, B. Sundararaju, Chem. Commun. 52 (2016) 1338-1341; (p) X.P. Chen, X.L. Cui, Y. Wu, J. Org. Lett. 18 (2016) 2411-2414; (q) X.P. Chen, X.L. Cui, Y.J. Wu, Org. Lett. 18 (2016) 3722-3725; (r) D. Kalsi, R.A. Laskar, N. Barsu, J.R. Premkumar, B. Sundararaju, Org. Lett. 18 (2016) 4198-4201; (s) R. Sharma, I. Kumar, R. Kumar, U. Sharma, Adv. Synth. Catal. 359 (2017) 3022-3028; (t) B. Wang, C.P. Li, H. Liu, Adv. Synth. Catal. 359 (2017) 3029-3034; (u) C. You, C. Pi, Y.J. Wu, X.L. Cui, Adv. Synth. Catal. 360 (2018) 4068-4072; (v) R. Sharma, R. Kumar, U. Sharma, J. Org. Chem. 84 (2019) 2786-2797; (w) B. Ghosh, A. Biswas, S. Chakraborty, R. Samanta, Chem. Asian J. 13 (2018) 2388-2392; (x) C. You, T.T. Yuan, Y.Z. Huang, et al., Org. Biomol. Chem.16 (2018) 4728-4733. |
| [15] |
(a) J. Kim, S. Kim, D. Kim, S. Chang, J. Org. Chem. 84 (2019) 13150-13158; (b) Z.H. Zhang, C. Pi, H. Tong, X.L. Cui, Y.J. Wu, Org. Lett. 19 (2017) 440-443. |
| [16] |
K. Konno, K. Hashimoto, H. Shirahama, T. Matsumoto, Heterocycles 24 (1986) 2169-2172. DOI:10.3987/R-1986-08-2169 |
| [17] |
D. Wenkert, R.B. Woodward, J. Org. Chem. 48 (1983) 283-289. DOI:10.1021/jo00151a001 |
| [18] |
X.M. Wang, D.G. Yu, F. Glorius, Angew. Chem. Int. Ed. 54 (2015) 10280-10283. DOI:10.1002/anie.201503888 |
 2020, Vol. 31
2020, Vol. 31