Propargylamines, a versatile class of frameworks, are widely used in medical chemistry and synthetic chemistry [1]. As such, different strategies have been developed so far to construct these compounds. In view of the synthetic efficiency and procedural simplicity of multicomponent reactions [2], transition metal-catalyzed A3-coupling of aldehydes, amines and alkynes is now widely employed [3]. Similarly, transition metal-catalyzed decarboxylative reactions, avoiding the use of organometallic reagents, have attracted significant attention [4]. As an alternative to classical A3 coupling, decarboxylative A3-type reaction, usually catalyzed by a transition metal such as copper, ruthenium or iridium is then performed to obtain the corresponding propargyl-amines [5]. However, these procedures involving the homogeneous copper salt as a catalyst generally suffered from reusability of catalyst [6]. Recently, heterogeneous catalysis for organic transformations has been of great interest. Solid-supported copper catalysts and copper nanoparticles currently being applied in the field of the A3 reaction possessed several advantages such as easy separation of reactants and reusability of catalyst than homogeneous catalysts [7]. As an alternative, the strategy by coupling copper catalyst with magnetic support has attracted great attention and has been strengthened in recent years. For instances, Aliaga et al. impregnated copper on magnetite as a catalyst for the multicomponent acetylene-Mannich reaction between terminal alkynes, secondary amines, and aldehydes [8a]. Song also utilized CuFe2O4 nanoparticles for catalyzing multicomponent synthesis of propargylamines [8b]. However, the specific surface area and active sites of copper-based magnetic catalysts is low, greatly decreasing the catalysis performance. Additionally, a series of copper-based nanocomposites such as Cu/HM, Chit@CuI, Cu@SiO2-NS and RGO@CuO have been developed for the application of decarboxylative A3 reactions (Scheme 1a) [9]. In these cases, the copper catalyst can be separated from the reaction system with centrifugation or filtration process, but the catalyst losing and time-energy consumption are inevitable. Nevertheless, the substrate scope of the process is quite limited thus far. Only propiolic acid and its derivatives, and amino acids were found to be the precursors for the synthesis of propargylamines. Therefore, introduction of a new carboxylic acid substrate to the decarboxylative A3-coupling, involving a new kind of magnetic copper catalyst with superior catalytic activity, cost economy as well as high stability loading for producing titled compounds, is still in high demand.
|
Download:
|
| Scheme 1. Heterogeneous copper-catalyzed decarboxylative A3-coupling. | |
The well-designed copper silicates have recently aroused considerable attention due to their excellent physical and chemical properties, highly abundant of copper ions, and low cost [10]. Srinivas et al. used two-dimensional mesoporous copper silicate catalyst with ordered two-dimensional pore structure for the efficient synthesis of propargylamines and their derivatives by A3-coupling [11]. Our group has recently synthesized a series of magnetic copper silicate with high specific surface area, showing exceptional performance as protein adsorbents [12]. Inspired by the above works, a creative idea is proposed to prepare a novel magnetic copper silicate composite which is used to catalyze the decarboxylative A3-coupling. Herein, we present a mild route to construct one dimensional (1D) magnetic copper silicate catalyst using a magnetic field-induced Stöber method followed by in-situ hydrothermal reaction with copper ions in ammonia buffer solution. This not only provides high content of copper ions of the copper silicates nanotubes, but also facilitating the recycle of catalyst with an external magnet. Catalytic performance tests demonstrate that the as-prepared Cu-based catalysts exhibit excellent activity and stability for introduction of a new carboxylic acid substrate to the decarboxylative A3-coupling reaction. The catalytic activity of Fe3O4@copper silicate is higher than that of Fe3O4@SiO2, Fe3O4, respectively. Moreover, the Fe3O4@CuSiO3 catalysts reported in this work hold great advantages over conventional Cu-based catalysts such as easy separation, higher activity etc. To the best of our knowledge, renewable magnetic copper catalysts have not been documented for decarboxylative A3-coupling of α-keto acids (Scheme 1b).
In order to achieve a heterogeneous copper-catalyzed decar-boxylative A3 reaction of α-keto acids, amines and alkynes, we started our studies with the preparation of a highly active, selective and recoverable Fe3O4-supported Cu catalyst. Firstly, the 1D Fe3O4@SiO2 nanochains were synthesized by a magnetic-induced modified Stöber method under moderate magnetic field. Once a magnetic field was applied in the process of silica coating, the magnetic NPs were brought together to form 1D Fe3O4@SiO2 nanochains by means of magnetic dipole-dipole interaction. SEM (Fig. 1A) and TEM (Fig. 1B) images confirmed that the magnetically assembled Fe3O4@SiO2 nanochains exhibited linear alignments with a smooth SiO2 shell about 30 nm in thickness. This indicates that the SiO2 layer plays vital role in the synthesis of 1D Fe3O4@SiO2 nanochains. Then the 1D Fe3O4@CuSiO3 nanochains were prepared by hydrothermal reaction between the 1D Fe3O4@SiO2 nanochains and mixture of aqueous CuCl2 in NH3-NH4Cl buffer system at 140 C for 12 h. The surface and morphologies of 1D Fe3O4@SiO2 nanochains and 1D Fe3O4@CuSiO3 nanochains were characterized from SEM and TEM techniques respectively, as shown in Figs. 1D and E. The as-obtained 1D hierarchical Fe3O4@CuSiO3 nanochains exhibit the hairy structures. Thanks for its one-dimensional hierarchical structures, the specific surface area of 1D hierarchical Fe3O4@CuSiO3 nanochains was as high as 179.33 m2/g, which was tested by the N2 adsorption-desorption isotherm (Fig. 1C). And pore size distribution was 5.46 nm, greatly facilitating the catalysis performance of organic molecules. X-ray photoelectron spectroscopy was also utilized to analyze the surface electronic states and the chemical elemental composition of the as-prepared composites. As shown in Fig. 1F, the detected signal peaks of Cu, Si, C, O elements were clearly observed in the survey XPS spectrum of 1D hierarchical Fe3O4@CuSiO3 hybrid. Owing to a loose shell thickness of copper silicate layer above the 10 nm (the limit detection of XPS) coating on Fe3O4 cores, so the signal of Fe element was almost disappeared. Notably, the atom ratio of copper ions is as high as 18.56%. In the Cu 2p core level spectrum, Cu 2p normally spin-orbit coupled into two peaks as well as several shake-up satellite peaks at 941.8 eV, 944 eV and 963 eV. As depicted in Fig. 1D, the binding energy of Cu 2p3/2 and Cu 2p1/2 at 935 eV and 955 eV could be addressed to copper ions, which confirmed the existence of CuSiO3 [13].

|
Download:
|
| Fig. 1. SEM (A, D) and TEM (B, E) images of 1D Fe3O4@SiO2 (A, B), 1D Fe3O4@CuSiO3 (D, E). (C) The N2 adsorption-desorption isotherms and pore size distribution curves (inset) of 1D Fe3O4@CuSiO3. (F) XPS of 1D Fe3O4@CuSiO3 and Cu 2p (inset). | |
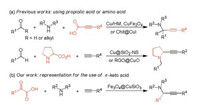
Subsequently, the catalytic activities were evaluated in a model reaction of phenylglyoxylic acid 1a, N-methylbenzylamine 2a and phenylacetylene 3a in the presence of Fe3O4@CuSiO3 (Table 1). To our delight, the desired product 4a was obtained in a yield of 34% using toluene as a solvent at 100 C for 16 h (entry 1). Other solvents such as DCE, THF, MeCN, dioxane and EtOH were screened, and EtOH provided the highest yield (entries 2–6). Further optimization showed that the experimental process of solvent-free significantly increased the yield to 74% (entry 7). The reaction afforded 83% yield under an Ar protecting atmosphere (entry 8). And then we controlled the amount of catalyst employed (entries 9–12). Indeed, the reaction proceeded smoothly in the presence of 3 mg (< 3 mol%) Fe3O4@CuSiO3 to produce 4a in excellent yield (entry 11), whereas previously reported gave almost the similar yield through the use of 20 mol% CuBr [5c]. These results indicated that heterogeneous copper catalyst played a significant role in enhancing the reaction. The importance of the catalyst Fe3O4@-CuSiO3 was again confirmed when the comparative experiments were carried out using Fe3O4@SiO2 (3 mg), or Fe3O4 (3 mg), or without catalyst (entries 13–15). Switching the reaction temperature to 85 ℃ or 115 ℃ resulted in a slightly lower yield (entries 16 and 17). Interesting, a best yield of the target product (94%) was achieved when the amount of 3a was increased to 0.8 mmol (entries 18 and 19). With conditions for the catalytic process have been identified, a gram-scale reaction was done to address the practical utility of this class of catalysts, giving a 93% yield of corresponding product 4a with the slight lower Fe3O4@CuSiO3 loading (entry 20).
|
|
Table 1 Optimization of the reaction conditions.a |
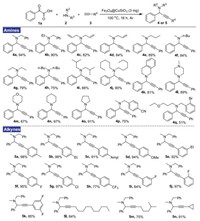
The substrate scope of decarboxylative A3-coupling, employing 1a with various amines 2 and alkynes 3, was surveyed (Scheme 2). Firstly, we started our investigations with the reaction of 15 different amine coupling partners including acyclic and cyclic aliphatic amines. It was found that in all cases the corresponding propargylamines 4a–4o were isolated in good to excellent yields. Additionally, functionalized substrates with CN or Br group were used to expand the application of this method, providing the corresponding products 4p and 4q. Then, a wide range of alkynes were investigated. Electron-rich as well as electron-poor groups were tolerated on the phenyl ring, delivering the desired compounds 5a–5k in 77%–98% yield. Notably, the reaction also worked well with aliphatic alkynes, giving the corresponding products 5l–5n in excellent yield.
|
Download:
|
| Scheme 2. Scopes of amines and alkynes. Reaction conditions: 1a (0.5 mmol), 2 (0.55 mmol), alkynes 3 (0.65 mmol), Fe3O4@CuSiO3 catalyst (3 mg) were stirred at 100 ℃ for 16 h under Ar atmosphere; isolated yield based on 1a. | |
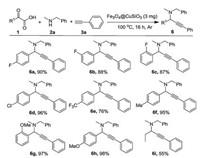
After these two initial evaluations, several α-keto acids were employed for the formation of the titled compounds (Scheme 3). In the case of the aromatic α-keto acids with different substituents, good to excellent yields of the corresponding products 6a–6h were observed. To our delight, 2-oxobutanoic acid also underwent this transformation to generate 55% yield of the desired propargylamine 6i.
|
Download:
|
| Scheme 3. Scopes of α-keto acids. Reaction conditions: α-keto acids 1 (0.5 mmol), 2a (0.55 mmol), 3a (0.65 mmol), Fe3O4@CuSiO3 catalyst (3 mg) were stirred at 100 ℃ for 16 h under Ar atmosphere; isolated yield based on 1. | |
Furthermore, the recyclability of Fe3O4@CuSiO3 catalyst was evaluated for the generation of product 4a under the standard conditions. After completion of reaction, the catalyst was separated by employing an external magnet, washed several times with ethyl acetate, and reused in a model reaction to provide 4a in 95% yield (Figs. 2A–C). This procedure was repeated for 5 more times as shown in Fig. 2D. The result indicated that its catalytic activity slightly lost, the isolated yield of the product was slowly decreased from 95% to 84%, owing to a little change of the morphologies of catalyst with the physical loss of catalyst amount after recycling and washing (see Supporting information: SEM image of the reused Fe3O4@CuSiO3).
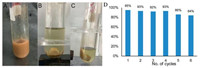
|
Download:
|
| Fig. 2. Photo of (A): Fe3O4@CuSiO3 dispersion in ethyl acetate; B, C: a magnet attracted the magnetic stirring bar and 1D Fe3O4@CuSiO3; (D) Recyclability of 1D Fe3O4@CuSiO3 catalyst for the synthesis of 4a. | |
Based on experimental observation and previous reports [5c, 14], a plausible mechanism for this 1D Fe3O4@CuSiO3 catalyzed decarboxylative A3-coupling is depicted in Fig. 3. Initially, the alkyne 3 is activated by a Fe3O4@CuSiO3 catalysis system, providing the corresponding the copper acetylide B through the intermediate A. Then, this species B reacts with α-keto acids 1 and amines 2 to produce the intermediate copper-complex C by a decarboxylative process, followed by the formation of the final products 4-6 along with the regeneration of the Fe3O4@CuSiO3-catalyst.

|
Download:
|
| Fig. 3. Proposed mechanism. | |
In summary, taking advantage of the magnetic induced Stöber method as well as the copper ion assisted hydrothermal process, one dimensional hierarchical Fe3O4@CuSiO3 was successfully fabricated. Owing to the large surface area, hierarchical copper silicate structure, and magnetic support, the as-prepared Fe3O4@CuSiO3 exhibits excellent performance for the decarboxylative A3-coupling. Moreover, compared with the other copper-based catalysts, the decarboxylative A3-coupling in this work is carried out under milder reaction conditions, and the resultant organic product can be easily separated with Fe3O4@CuSiO3 catalysts using an external magnetic field, which greatly facilitate the process of purification and the recycle of magnetic catalyst. We believe that this kind of magnetic catalyst could be extended to other organic reaction systems which are catalyzed by transition metal ions.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (Nos. 21601121, 21305086), the Natural Science Foundation of Shanghai (No. 18ZR1416400). We also acknowledge the support of the Shanghai University of Engineering Science (Nos. 201810856017, A1-0601-19-01017), and the Opening Project of Shanghai Key Laboratory of Chemical Biology for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.004.
| [1] |
(a) J.J. Chen, D.M. Swope, J. Clin. Pharmacol. 45 (2005) 878-894; (b) M. Baranyi, P.F. Porceddu, F. Gölöncse'r, et al., Mol. Neurodegener.11 (2016) 1-21; (c) I. Bolea, A. Gella, M. Unzeta, J. Neural Transm. 120 (2013) 893-902; (d) F.T. Zindo, J. Joubert, S.F. Malan, Future Med. Chem. 7 (2015) 609-629; (e) K. Lauder, A. Toscani, N. Scalacci, D. Castagnolo, Chem. Rev. 117 (2017) 14091-14200; (f) V.A. Peshkov, O.P. Pereshivko, A.A. Nechaev, A.A. Peshkov, E.V. Vander Eycken, Chem. Soc. Rev. 47 (2018) 3861-3898. |
| [2] |
(a) X.X. Sun, C. Li, Y.Y. He, et al., Adv. Synth. Catal. 359 (2017) 2660-2670; (b) Y.M. Wang, H.H. Zhang, C. Li, T. Fan, F. Shi, Chem. Commun. 52 (2016) 1804-1807; (c) X.X.Sun, H.H.Zhang, G.H.Li, Y.Y.He, F.Shi, Chem.Eur.J.22 (2016)17526-17532. |
| [3] |
(a) C. Wei, Z. Li, C.J. Li, Synlett (2004) 1472-1483; (b) L. Zani, C. Bolm, Chem. Commun. 38 (2006) 4263-4275; (c) V.A. Peshkov, O.P. Pereshivko, E. Van der Eycken, Chem. Soc. Rev. 41 (2012) 3790-3807; (d) D. Seidel, Org. Chem. Front. 1 (2014) 426-429. |
| [4] |
(a) R. Shang, L. Liu, Sci. China Chem. 54 (2011) 1670-1687; (b) N. Rodríguez, L.J. Goossen, Chem. Soc. Rev. 40 (2011) 5030-5048; (c) J.D.Weaver, A.Recio, A.J.Grenning, J.A.Tunge, Chem.Rev.111(2011)1846-1913; (d) J. Schwarz, B. König, Green Chem. 20 (2018) 323-361; (e) H.D. Feng, H.H. Jia, Z.H. Sun, Adv. Synth. Catal. 357(2015) 2447-2452; (f) H.D. Feng, H.H. Jia, Z.H. Sun, J. Org. Chem. 79 (2014) 11812-17181. |
| [5] |
(a) F.L. Vaillant, T.J. Courant, Angew. Chem. Int. Ed. 54 (2015) 11200-11204; (b) H.Zhang, P.X. Zhang, M.Jiang, H.J.Yang, H.Fu, Org. Lett.19 (2017)1016-1019; (c)H.D.Feng, D.S.Ermolat'ev, G.H.Song, E.VanderEycken, J.Org.Chem.76 (2011) 7608-7613; (d) P.F. Zhao, H.D. Feng, H.R. Pan, Z.H. Sun, M.C. Tong, Org. Chem. Front. 4 (2017) 37-41. |
| [6] |
(a) H.P. Bi, L. Zhao, Y.M. Liang, C.J. Li, Angew. Chem. Int. Ed. 48 (2009) 792-795; (b) H.P. Bi, Q. Teng, M. Guan, et al., J. Org. Chem. 75 (2010) 783-788; (c) D. Chen, P. Huang, Y. Yu, et al., Chem. Commun. 47 (2011) 5801-5803; (d) H.D. Feng, D.S. Ermolat'ev, G.H. Song, E. Van der Eycken, J. Org. Chem. 77 (2012) 5149-5154; (e) H.D. Feng, D.S. Ermolat'ev, G.H. Song, E. Van der Eycken, Org. Lett.14 (2012) 1942-1945. |
| [7] |
(a) I. Luz, F.X.L. Xamena, A. Corma, J. Catal. 285 (2012) 285-291; (b) G. Bosica, R. Abdilla, J. Mol. Catal. A: Chem. 426 (2017) 542-549; (c) A.V. Nakhat, G.D. Yada, Mol. Catal. 451(2018) 209-219. |
| [8] |
(a) M.J. Aliaga, D.J. Ramón, M. Yus, Org. Biomol. Chem. 8 (2010) 43-46; (b) J.Y. Zhang, X. Huang, Q.Y. Shen, J.Y. Wang, G.H. Song, Chin. Chem. Lett. 29 (2018) 197-200. |
| [9] |
(a) U.C. Rajesh, U. Gulati, D.S. Rawat, ACS Sustainable Chem. Eng. 4 (2016) 3409-3419; (b) P. Kaur, B. Kumar, V. Kumar, R. Kumar, Tetrahedron Lett. 59 (2018) 1986-1991; (c) U. Gulati, U.C. Rajesh, N. Bunekar, D.S. Rawat, ACS Sustainable Chem. Eng. 5 (2017) 4672-4682; (d) U. Gulati, U.C. Rajesh, D.S. Rawat, ACS Sustainable Chem. Eng. 6 (2018) 10039-10051. |
| [10] |
(a) Y. Li, Y. Lu, C. Zhao, et al., Energy Storage Mater. 7 (2017) 130-151; (b) K.M. Kwok, S.W.D. Ong, L. Chen, H.C. Zeng, ACS Appl. Mater. Interface 9 (2017) 37210-37222. |
| [11] |
M. Srinivas, P. Srinivasu, S.K. Bhargava, M.L. Kantam, Catal. Today 208 (2013) 66-71. DOI:10.1016/j.cattod.2013.02.006 |
| [12] |
(a) M. Zhang, Y.T. Wang, Y.W. Zhang, et al., Appl. Surf. Sci. 375 (2016) 154-161; (b) Y. Zhang, M. Zhang, J. Yang, L.J. Zheng, J.L. Xu, J. Alloys Compd. 695 (2017) 3256-3266; (c) M. Zhang, B.Y. Wang, W.Z. Li, W.J. Gan, Dalton Trans. 45 (2016) 922-927; (d) M. Zhang, T. Miao, J. Zheng, et al., Microporous Mesoporous Mater. 286 (2019) 207-213. |
| [13] |
J.W. Liu, J. Cheng, R.C. Che, et al., ACS Appl. Mater. Inter. 5 (2013) 2503-2509. DOI:10.1021/am3030432 |
| [14] |
J. Choi, J. Lim, F.M. Irudayanathan, et al., Asian J. Org. Chem. 5 (2016) 770-777. DOI:10.1002/ajoc.201600109 |
 2020, Vol. 31
2020, Vol. 31