b College of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou 225002, China
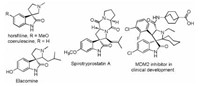
Spirooxindole is an important heterocyclic nucleus with broad biological activity and applications for pharmaceutical lead discovery [1-4]. Among the various spirooxindoles, the spiroox-indole-pyrrolidine is one of the most frequently encountered in natural alkaloids and is often considered as attractive templates for drug discovery [5-8]. They have been widely studied on the antiviral, antibacterial, anti-cancer and cholinesterase inhibitory activities, etc. (Fig. 1) [9-11]. As a consequence, many efficient synthetic procedures have been developed for the preparation of the structurally diverse spirooxindole-pyrrolidine derivatives [12-18]. The 1, 3-dipolar cycloaddition reaction is one of the most important method for the construction of spirooxindole-pyrroli-dines [19-23]. For this purpose, the cycloaddition reaction of various azomethine ylides with active electron-deficient alkenes has become the convenient method for synthesis of various spirooxindole-pyrrolidines [24-27]. On the other hand, the electron-deficient alkynes were also employed to react with azomethine ylides generating from reaction of isatins with suitable amines in 1, 3-dipolar cycloaddition [28-36]. In recent years, dialkyl hex-2-en-4-ynedioates have emerged as new reactive electron-deficient alkynes in various cycloaddition reactions [36-40]. Dialkyl hex-2-en-4-ynedioates can be easily prepared in nearly quantitative yields from base-catalysed dimerization of alkyl propiolates under mild reaction conditions [41-49]. We have also developed several domino reactions by employing nucleophilic triphenylphosphine addition to electron-deficient alkynes as key protocol for the efficient construction of diverse polycyclic and spiro compounds [50-55]. In this paper, we wish to report the 1, 3-dipolar cycloaddition reaction of dimethyl hex-2-en-4-ynedioate with azomethine ylides derived from reaction of L-proline with various isatins in methanol and selectively synthesis of function-alized spiro[indoline-3, 3'-pyrrolizines].
|
Download:
|
| Fig. 1. Representative of bioactive natural and synthetic products having spirooxindole-pyrrolidine. | |
According to the previously established reaction conditions for the preparing of the spiro[indoline-3, 3'-pyrrolizine] [28-33], a mixture of L-proline, isatin and dimethyl hex-2-en-4-ynedioate in methanol was stirred at room temperature. TLC analysis showed that the reaction was completed in 24 h. After workup, the spiro compound 1a and the spiro compound 2a were obtained in 62% and 20% yields, which can be separated out by column chromatography. The structural analysis indicated that the spiro compound 1a has a unit of methyl acrylate, which was clearly come from the reaction of 1, 3-dipolar cycloaddition of azomethine ylide with the C≡C triple bond in dimethyl hex-2-en-4-ynedioate. On the other hand, the [3 + 2] reaction of the methylene ylide with the C=C double bond in dimethyl hex-2-en-4-ynedioate afforded the spiro compound 2a. The much higher yield of 1a to that of 2a showed that the C≡C triple bond has higher reactivity than that of C=C double bond in dimethyl hex-2-en-4-ynedioate. In order to increase the selectivity of the reaction, the three-component reaction was carried out in ethanol, acetonitrile at room temperature and at the elevated temperature. But similar yields of the spiro compounds 1a and 2a were obtained. Then various substituted isatins were employed in the reaction. The results are summarized in Table 1 (Experimental section, 1H and 13C NMR spectra for all new compounds are listed in Supporting information). It can be seen that the reaction usually resulted in the spiro compounds 1a-1n as main product and the spiro compound 2a-2n as minor products. In some cases, the minor spiro compounds were not isolated due to too lower yields.
|
|
Table 1 Synthesis of spiro[indoline-3, 3'-pyrrolizines] via cycloaddition reaction.a |
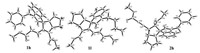
The molecular structures of the obtianed spiro compounds were fully characterized by IR, HRMS, 1H and 13C NMR spectra. The single crystal structures of the compounds 1h, 1l and 2h (Fig. 2) were succesfully detemined by X-ray diffraction method. Crystal-lographic data 1h (CCDC No. 1588730), 1l (CCDC No. 1588731), 2h (CCDC No. 1588732) have been deposited at the Cambridge Crystallographic Database Centre. In the compounds 1h and 1l, it can be seen that the acrylate unit exists in E-configuration. In the molecular structure of the compound 2h, methyl carboxylate and methyl propiolate exist on the trans-position. On the other hand, the methyl carboxylate exist on the cis-position to the phenyl group of the oxindole scaffold. Thus, relative configurations of the obtained spiro compounds were clearly elucidated on the basis of NMR spectra and the single crystal structures.
|
Download:
|
| Fig. 2. Molecular structures of the compounds 1h, 1l and 2h. | |
For explaining the formation of the two kinds of spiro compounds, a plausible reaction mechanism was proposed on the basis of the similar 1, 3-dipolar cycloaddition reaction (Scheme 1). At first, the condensation of L-proline with isatin afforded known intermediate azomethine ylide (A). Because the C≡C triple bond and C=C double bond connected to the electron-withdrawing ester group in dimethyl hex-2-en-4-ynedioate, both of them could react as the reactive 1, 3-dipolarophiles. Thus, the 1, 3-dipolar cycloaddition of azomethine ylide (A) with more reactive C≡C triple bond according to the path a gave the spiro compound 1 as main product. On the other hand, the 1, 3-dipolar cycloaddition of azomethine ylide (A) with less reactive C=C double bond according to the path b resulted in the spiro compound 2 as minor product. Due to the concerted reaction of 1, 3-dipolar cycloaddition, the two substituents retained the trans-configuration in the newly-formed ring of pyrrole in the spiro compound 2. Thus, dimethyl hex-2-en-4-ynedioate provided an ideal example for comparing the reactivity of electron-deficient C=C double bond and C≡C triple bond as the active 1, 3-dipolarophiles in cycloaddition reaction. Our experimental results clearly revealed that the electron-deficient alkyne has much high reactivity than that of electron-deficient alkene in 1, 3-dipolar cycloaddition reaction.

|
Download:
|
| Scheme 1. Proposed formation mechanism for spiro compounds. | |
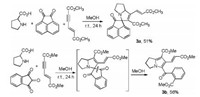
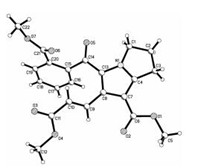
For developing the scope of this reaction, acenaphthylene-1, 2-dione was also employed to react with proline and dimethyl hex-2-en-4-ynedioate in methanol at room temperature. The spiro compound 3a was successfully obtained in 51% yields, in which the C=C double bond was remained in the molecule (Scheme 2). On the other hand, the reaction with ninhydrin resulted in the product 3b. It was obviously produced from the further ring-opening of the initially formed spiro[indene-2, 3'-pyrrolizine] via [3+2] cycloaddition reaction. The single crystal structure of the compound 3b (Fig. 3) has been determined by X-ray diffraction method. Crystallographic data 3b (CCDC No. 1972418) has been deposited at the Cambridge Crystallographic Database Centre.
|
Download:
|
| Scheme 2. Reactions with acenaphthylene-1, 2-dione and ninhydrin. | |
|
Download:
|
| Fig. 3. Molecular structures of the compounds 3b. | |
For demonstrating the synthetic values of this three-compo-nent reaction, the variety of amino acids were also examined.Therefore, thioproline, 4-hydroxyproline and sarcosine were also employed to replace proline in the three-component reaction under same reaction conditions (Scheme 3). The similar spiro compounds 4a-4c were formed as main products. In each case, the C≡C triple bond took part in the reaction and the C=C double bond was remained in the molecule. These results indicated again the electron-deficient C≡C triple bond is more reactive than the electron-deficient C=C double bond in 1, 3-dipolar cycloaddition reaction.

|
Download:
|
| Scheme 3. Reactions with other amino acids. | |
In summary, we have investigated 1, 3-dipolar cycloaddition reaction of dimethyl hex-2-en-4-ynedioate with azomethine ylides derived from reaction of L-proline with various isatins in methanol. This reaction successfully provided an convenient synthetic protocol for functionalized spiro[indoline-3, 3'-pyrroli-zine]acrylates as main products and spiro[indoline-3, 3'-pyrroli-zine]propiolates as minor products. The stereochemistry of the spiro compounds were clearly elucidated by determination of several single crystal structures. Additionally, this reaction provided an example to reveal the relative reactivity of the electron-deficient alkyne and electron-deficient alkene in 1, 3-dipolar cycloaddition reaction.
Declaration of competing interestWe declared there is no conflict of interest in this paper.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.12.028.
| [1] |
M.M.M. Santos, Tetrahedron 70 (2014) 9735-9757. DOI:10.1016/j.tet.2014.08.005 |
| [2] |
B. Yu, D.Q. Yu, H.M. Liu, Eur. J. Med. Chem. 97 (2015) 673-698. DOI:10.1016/j.ejmech.2014.06.056 |
| [3] |
M. Kaur, M. Singh, N. Chadha, O. Silakari, Eur. J. Med. Chem. 123 (2016) 858-894. DOI:10.1016/j.ejmech.2016.08.011 |
| [4] |
N. Ye, H.Y. Chen, E.A. Wold, P.Y. Shi, J. Zhou, ACS Infect. Dis. 2 (2016) 382-392. DOI:10.1021/acsinfecdis.6b00041 |
| [5] |
G.M. Ziarani, R. Moradi, N. Lashgari, Tetrahedron 74 (2018) 1323-1353. DOI:10.1016/j.tet.2018.01.025 |
| [6] |
S.D. Lotesta, A.P. Marcus, Y.J. Zheng, et al., Bioorg. Med. Chem. 24 (2016) 1384-1391. DOI:10.1016/j.bmc.2016.02.014 |
| [7] |
A.B. Dounay, L.E. Overman, Chem. Rev. 103 (2003) 2945-2963. DOI:10.1021/cr020039h |
| [8] |
G.S. Singh, Z.Y. Desta, Chem. Rev. 12 (2012) 6104-6155. |
| [9] |
N.R. Ball-Jones, J.J. Badillo, A.K. Franz, Org. Biomol. Chem. 10 (2012) 5165-5181. DOI:10.1039/c2ob25184a |
| [10] |
D.Q. Yu, H.M. Liu, Eur. J. Med. Chem. 97 (2015) 673-698. DOI:10.1016/j.ejmech.2014.06.056 |
| [11] |
C.V. Galliford, K.A. Scheidt, Angew. Chem. Int. Ed. 46 (2007) 8748-8758. DOI:10.1002/anie.200701342 |
| [12] |
Y.Y. Liu, H. Wang, J.P. Wan, Asian J. Org. Chem. 2 (2013) 374-386. DOI:10.1002/ajoc.201200180 |
| [13] |
Z.Y. Cao, Y.H. Wang, X.P. Zeng, J. Zhou, Tetrahedron 70 (2014) 2406-2415. DOI:10.1016/j.tet.2014.02.023 |
| [14] |
D.Q. Cheng, Y. Ishihara, B. Tan, et al., ACS Catal. 4 (2014) 743-762. DOI:10.1021/cs401172r |
| [15] |
R.G. Redkin, V.V. Lipson, D.V. Atamanuk, Mol. Divers. 20 (2016) 299-344. DOI:10.1007/s11030-015-9629-8 |
| [16] |
Z. Cao, Q. Zhu, Y.W. Lin, et al., Chin. Chem. Lett. 30 (2019) 1237-1240. DOI:10.1016/j.cclet.2019.04.033 |
| [17] |
L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 2132-2138. DOI:10.1016/j.cclet.2019.09.041 |
| [18] |
C. Wu, L.H. Lu, A.Z. Peng, et al., Green Chem. 20 (2018) 3683-3688. DOI:10.1039/C8GC00491A |
| [19] |
G. Palmisano, R. Annunziata, G. Papeo, M. Sisti, Tetrahedron Asymmetry 7 (1996) 1-4. |
| [20] |
I. Fejes, M. Nyerges, A. Szollosy, et al., Tetrahedron 57 (2001) 1129-1137. DOI:10.1016/S0040-4020(00)01085-1 |
| [21] |
A.R.S. Babu, R. Raghunathan, Tetrahedron Lett. 48 (2007) 305-308. DOI:10.1016/j.tetlet.2006.11.012 |
| [22] |
M. Ghandi, A. Yari, S.J.T. Rezaei, A. Taheri, Tetrahedron Lett. 50 (2009) 4724-4726. DOI:10.1016/j.tetlet.2009.06.033 |
| [23] |
R. Jain, K. Sharma, D. Kumar, Tetrahedron Lett. 53 (2012) 1993-1997. DOI:10.1016/j.tetlet.2012.02.029 |
| [24] |
S. Lanka, S. Thennarasu, P.T. Perumal, Tetrahedron Lett. 53 (2012) 7052-7055. DOI:10.1016/j.tetlet.2012.10.061 |
| [25] |
A.S. Girgis, J. Stawinski, N.S.M. Ismail, H. Farag, Eur. J. Med. Chem. 47 (2012) 312-322. DOI:10.1016/j.ejmech.2011.10.058 |
| [26] |
W. Ren, Q. Zhao, C. Zheng, et al., Molecules 21 (2016) 113. DOI:10.3390/molecules21010113 |
| [27] |
S. Basu, C. Mukhopadhyay, Eur. J. Org. Chem. 12 (2018) 1496-1506. |
| [28] |
S. Kolle, B. Shivalinga, D.S. Barak, et al., ACS Omega 4 (2019) 5617-5629. DOI:10.1021/acsomega.9b00396 |
| [29] |
R.T. Pardasani, P. Pardasani, V. Chaturvedi, et al., Heteroatom Chem. 14 (2003) 36-41. DOI:10.1002/hc.10063 |
| [30] |
S.N. Singh, S. Regati, A.K. Paul, et al., Tetrahedron Lett. 54 (2013) 5448-5452. DOI:10.1016/j.tetlet.2013.07.126 |
| [31] |
G. Bhaskar, Y. Arun, C. Balachandran, et al., Eur. J. Med. Chem. 51 (2012) 79-91. DOI:10.1016/j.ejmech.2012.02.024 |
| [32] |
M. Narayanarao, L. Koodlur, S. Gopal, etal., Synth.Commun. 48 (2018) 2441-2451. DOI:10.1080/00397911.2018.1508722 |
| [33] |
P.R. Mali, P.K. Shirsat, N. Khomane, et al., ACS Comb. Sci. 19 (2017) 633-639. DOI:10.1021/acscombsci.7b00044 |
| [34] |
S. Basu, U. Kayal, S. Maity, et al., ChemistrySelect 3 (2018) 12755-12763. |
| [35] |
F. Yang, J. Sun, H. Gao, C.G. Yan, RSC Adv. 5 (2015) 32786-32794. DOI:10.1039/C5RA04102C |
| [36] |
J. Cao, F. Yang, J. Sun, et al., J. Org. Chem. 84 (2019) 622-635. DOI:10.1021/acs.joc.8b02457 |
| [37] |
S. Li, Y. Liu, B. Huang, et al., ACS Catal. 7 (2017) 2805-2809. DOI:10.1021/acscatal.7b00030 |
| [38] |
P.V. Ramachandran, M.T. Rudd, M.V.R. Reddy, Tetrahedron Lett. 46 (2005) 2547-2549. DOI:10.1016/j.tetlet.2005.02.098 |
| [39] |
L.H. Zhou, X.Q. Yu, L. Pu, Tetrahedron Lett. 51 (2010) 425-427. DOI:10.1016/j.tetlet.2009.11.048 |
| [40] |
S.F. Duan, D.K. Sinha-Mahapatra, J.W. Herndon, Org. Lett. 10 (2008) 1541-1544. |
| [41] |
F. Puenner, G. Hilt, Chem. Commun. (Camb.) 48 (2012) 3617-3619. DOI:10.1039/c2cc30777d |
| [42] |
S. Raju, P. Annamalai, F.W. Chan, et al., Synthesis 49 (2017) 5007-5016. DOI:10.1055/s-0036-1588501 |
| [43] |
S.F. Jin, Y.J. Niu, C.J. Liu, et al., J. Org. Chem. 82 (2017) 9066-9074. DOI:10.1021/acs.joc.7b01561 |
| [44] |
S. Sakthivel, A. Sharma, R Balamurugan, Eur. J. Org. Chem. (2017) 3941-3946. |
| [45] |
B. Dhakal, L.S.R. Gamage, Y.S. Zhang, J.W. Herndon, Tetrahedron Lett. 58 (2017) 1403-1407. DOI:10.1016/j.tetlet.2017.02.070 |
| [46] |
J.H. Choi, C.M. Park, Adv. Synth. Catal. 360 (2018) 3553-3562. DOI:10.1002/adsc.201800734 |
| [47] |
J.C. Deng, S.C. Chuang, Org. Lett. 13 (2011) 2248-2251. DOI:10.1021/ol200527t |
| [48] |
P.Y. Tseng, S.C. Chuang, Adv. Synth. Catal. 355 (2013) 2165-2171. DOI:10.1002/adsc.201300255 |
| [49] |
J.C. Deng, W.Y. Chen, C.Y. Zhu, et al., Adv. Synth. Catal. 357 (2015) 1453-1462. DOI:10.1002/adsc.201401134 |
| [50] |
A.J. Wu, P.Y. Tseng, W.H. Hsu, et al., Org. Lett. 18 (2016) 224-227. DOI:10.1021/acs.orglett.5b03293 |
| [51] |
Y. Han, Y.J. Sheng, C.G. Yan, Org. Lett. 16 (2014) 2654-2657. DOI:10.1021/ol5008394 |
| [52] |
G. Hui, J. Sun, C.G. Yan, Synthesis 46 (2014) 2327-2332. DOI:10.1055/s-0033-1339120 |
| [53] |
Y. Han, W.J. Qi, Y.J. Shen, et al., Tetrahedron Lett. 56 (2015) 5196-5198. DOI:10.1016/j.tetlet.2015.07.071 |
| [54] |
W.J. Qi, Y. Han, C.G. Yan, Tetrahedron 72 (2016) 5057-5063. DOI:10.1016/j.tet.2016.06.020 |
| [55] |
C.Z. Liu, Y. Han, W.J. Qi, et al., Heterocyclic Commun. 22 (2016) 301-306. |
 2020, Vol. 31
2020, Vol. 31 


