b School of Chemistry and Chemical Engineering, Nantong University, Nantong 226019, China
Rotaxane [1], usually constructed from macro-cyclic wheels and linear axles, have been attracted great interesting due to their unique interlocked structures and wide application in various areas such as molecular machines, stimuli-responsive materials, information storage and supramolecular catalysts [2]. The macrocyclic host and its noncovalent interaction are keys for the construction rotaxanes [3]. During the past decades, crown ethers, cyclodextrins, calix[n]arenes and cucurbiturils have been used as wheels to fabricate [n]rotaxanes successfully [4].
Pillar[5]arenes, which were first prepared by Ogoshi in 2008 [5], are a very "smart" type of newly supramolecular hosts and have been widely investigated in recent years [6, 7]. The symmetrical pillar-like topology and π-electron rich cavity endow pillar[5]arenes as ideal platforms for host-guest chemistry [8]. Pillar[5]arene-based [1]rotaxanes and [2]rotaxanes have been attracted much attention and widely investigated [9]. For example, Prof. Yang and co-workers synthesized a pillar[5]arene-based [1]rotaxane from a self-included pseudo[1]rotaxane direct reacting with a stopper. What is more, this [1]rotaxane proved to be a good catalyst for Knoevenagel reaction in CHCl3 [9a]. Prof. Huang and co-workers prepared a pillar [5]arene/imidazolium [2]rotaxane, and found this [2]rotaxane showed solvent- and thermo-driven molecular motions [9b].
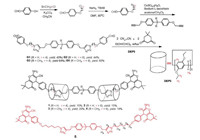
However, higher-ordered [n]rotaxanes (n ≥ 3) based on pillar[5]arene remain rarely studied limited by the poor synthetic yield, despite their fascinating structure and potential applications in molecular devices [10]. Herein, in this study, we designed and conveniently constructed four pillar[5]arene-based [3]rotaxanes (1-4) from two pillar[5]arene wheels and a linear guest in high yields with one-pot multicomponent reaction. As shown in Scheme 1, linear guests G1-G4 containing two benzaldehyde groups were prepared in 3 steps from p-hydroxybenzaldehyde with yields about 80% firstly (Figs. S1-S12 in Supporting information). Then, 1, 4-diethoxypillar[5]arene (DEP5) and aliphatic chain axel G1-G4 were stirred in chloroform/ethanol mixture at room temperature overnight, which enable the axel threaded into the cavity of pillar[5]arene. At last, the base catalyzed three-component reaction of dimedone, malononitrile and benzaldehyde was used as the stopper reaction. The three-component reaction was carried out in refluxing ethanol in the presence of piperidine for 3 h. After workup, the expected pillar[5]arene-based [3]rotaxanes 1-4 were successfully obtained in moderate yields (Figs. S13-S24 in Supporting information). For comparison, "dumbbell" molecule 5 was also prepared in the absence of DEP5 (Scheme 1).
|
Download:
|
| Scheme 1. A synthetic route to guests G1-G4 and [3]rotaxanes 1–4. | |
Fig. 1 showed the typical 1H NMR spectra of dumbbell 5, wheel DEP5 and [3]rotaxane 4. The -CH2- protons Hc, Hd, He, Hf, Hh and Hb in triazole of 4 showed clearly upfield shifts in comparison with those of compound 5 due to the encapsulation induced shieling effects [11]. On the other hand, the protons Hi, Hj and Hk of the wheel DEP5 shifted downfield compared with free pillar[5]arene, which was due to the deshielding effects. It should be pointed that the 1H NMR experiments were investigated in highly polar DMSO-d6, indicating that the obtained stopper is bulky enough for the [3]rotaxane.

|
Download:
|
| Fig. 1. 1H NMR spectra (room temperature, 400 MHz, DMSO-d6) of 2.00 mmol/L DEP5 (a), 2.00 mmol/L 4 (b), and 2.00 mmol/L axle 5 (c). | |
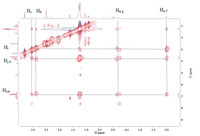
Furthermore, 2D NOESY spectrum of [3]rotaxane 4 in DMSO-d6 showed clearly NOE correlation signals between protons Hi, Hj, Hk, and Hl of the wheel pillar[5]arene and protons Hb, Hc, Hd, He, Hf, Hg, and Hh of the linear axle (Fig. 2). In addition, mass spectrometry also confirmed the formation of [3]rotaxane. In the mass spectrum of [3]rotaxane 4 (Fig. S24), a base peak at m/z 1479.8145 (100%) corresponding to [M+2H]2+ was observed. All the above NMR and mass investigations led us to conclude the formation of [3]rotaxane. [3]Rotaxanes 1–3 were also confirmed by the similar NMR and mass studies (Figs. S13-S21).
|
Download:
|
| Fig. 2. Partial 2D NOESY NMR spectrum of [3]rotaxane 4. | |
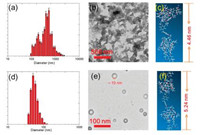
Considering the symmetrical pillar-like, rigid architecture incorporated in [3]rotaxane 4, we wondered the potential selfassembly behavior of 4 in water. [3]Rotaxane 4 was dissolved in DMF firstly (10-3 mol/L), then 1.00 mL solution of 4 was injected into 99 mL water under vigorous stirring. Dynamic light scattering (DLS) experiment performed with a 1.00 × 10-5 mol/L aqueous solution of 4 over a scattering angle of 90° showed a broad size distribution (Fig. 3a). The average hydrodynamic diameter (Dh) of 4 was observed to be about 500 nm. Then we used transmission electron microscopy (TEM) to investigate the self-assembly behaviors of [3]rotaxane in water. As shown in Fig. 3b, 4 self-assembled into irregular solid particles, whose average diameter was about 500 nm, much larger than the length of the 4, indicating that the particles self-assembled from tremendous molecules fusing together (Fig. 3c).
|
Download:
|
| Fig. 3. (a) DLS data of 4-based aggregates in water. (b) TEM image of 4-based aggregates in water. (c) Minimize energy structure of 4 (calculated by Gaussian 09 B. 01/B3LYP/6-31 G (d, p)). (d) DLS data of 4-based assemblies in water after addition of H+. (e) TEM image of 4-based assemblies in water after addition of H+. (f) Minimize energy structure of 4-H (calculated by Gaussian 09 B. 01/B3LYP/6-31 G (d, p)). | |
Inspired by the amino groups and triazole units can be protonated by H+, we then investigated the pH-responsive property of 4 in water. When we added 4 equiv. of HCl into the particles' solution, DLS showed that average hydrodynamic diameter (Dh) of 4-H was changed to 40 nm with narrow distribution (Fig. 3d). TEM confirmed that the large particles transformed into vesicles with diameter about 40 nm (Fig. 3e). What is more, the thickness of the vesicle' wall is about 10 nm, indicating its bilayer (Fig. 3f). This transformation is due to that when 4 were protonated, it became an amphiphilic compound, so it can self-assemble into regular vesicles through hydrophilic-hydrophobic interactions.
In conclusion, four pillar[5]arene based [3]rotaxanes (1-4) involving two pillar[5]arene (P5) rings and a dumbbell-shaped component were synthesized successfully via multicomponent reaction. The structures of [3]rotaxanes were characterized and confirmed by 1H NMR, 13C NMR, NOESY and HR-ESI-MS technologies. Furthermore, take 4 as example, due to the amino groups and triazole units incorporated in 4 can be protonated by H+, when addition of 4 equiv. of HCl into the aqueous solution of 4, well-defined vesicles with diameter about 40 nm were observed. We hope that pillar[5]arene based [3]rotaxanes may have potential applications in drug delivery systems and molecular devices.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21871227, 21801139), Natural Science Foundation of Jiangsu Province (No. BK20180942), Natural Science Foundation of Nantong University for High-Level Talent (No. 03083004).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.041.
| [1] |
(a) K.D. Hanni, D.A. Leigh, Chem. Soc. Rev. 39 (2010) 1240-1251; (b) M.D. Lankshear, P.D. Beer, Acc. Chem. Res. 40 (2007) 657-668; (c) T. Ikeda, M. Higuchi, D.G. Kurth, J. Am. Chem. Soc. 131 (2009) 9158-9159; (d) M. He, L. Chen, B. Jiang, et al., Chin. Chem. Lett. 30 (2019) 131-134. |
| [2] |
(a) T. Ogoshi, D. Yamafuji, T. Aoki, T.A. Yamagishi, Chem. Commun. (Camb.) 48 (2012) 6842-6844; (b) Y. Yao, X.J. Wei, Y. Cai, et al., J. Colloid Interf. Sci. 533 (2019) 42-46. |
| [3] |
E.M.G. Jamieson, F. Modicom, S.M. Goldup, Chem. Soc. Rev. 47 (2018) 5266-5311. DOI:10.1039/C8CS00097B |
| [4] |
(a) Y. Liu, Q. Zhang, W.H. Jin, et al., Chem. Commun. (Camb.) 54 (2018) 10642-10645; (b) T.G. Zhan, H.H. Yin, S.T. Zheng, et al., Chem. Commun. (Camb.) 54 (2018) 9356-9359; (c) G. Gholami, K. Zhu, G. Baggi, et al., Chem. Sci. 8 (2017) 7718-7723; (d) W. Wu, S. Song, X. Cui, et al., Chin. Chem. Lett. 29 (2018) 95-98; (e) R. Zhang, C. Wang, R. Long, et al., Front. Chem. 7 (2019) 508. |
| [5] |
T. Ogoshi, S. Kanai, S. Fujinami, et al., J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [6] |
(a) S. Sun, D. Lu, Q. Huang, et al., Colloids Interface Sci. Commun. 533 (2019) 42-46; (b) S. Sun, M. Geng, L. Huang, et al., Chem. Commun. (Camb.) 54 (2018) 13006-13009; (c) K. Jie, Y. Zhou, E. Li, et al., Acc. Chem. Res. 51 (2018) 2064-2072; (d) Y. Zhang, W. Zhu, X. Huang, et al., ACS Sust. Chem.Eng. 6 (2018)16597-16606; (e) E. Li, K. Jie, Y. Zhou, et al., J. Am. Chem. Soc. 140 (2018) 15070-15079; (f) J.R. Wu, A. Mu, B. Li, et al., Angew. Chem. Ing. Ed. 57 (2018) 9853-9858; (g) M. Zuo, W. Qian, Z. Xu, et al., Small 14 (2018) 1801942; (h) L.L. Zhao, Y. Han, C.G. Yan, Chin. Chem. Lett. 31 (2020) 81-83; (i) C.B. Yin, Y. Han, G.F. Huo, et al., Chin. Chem. Lett. 28 (2017) 431-436; (j)J.Chen, Y.Wang, C.Wang, etal., Chem.Commun.(Camb.)55 (2019)6817-6826. |
| [7] |
(a) T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276; (b) T. Xiao, W. Zhong, L. Xu, X.Q. Sun, X.Y. Hu, L. Wang, Org. Biomol. Chem. 17 (2019) 1336-1350; (c) Y. Wang, T. Liu, J. Jiang, et al., Dalton Trans. 48 (2019) 6333-6336. |
| [8] |
(a) Q. Wang, L. Tian, J. Xu, et al., Chem. Commun. (Camb.) 54 (2018) 10328-10331; (b) K. Yang, K. Yang, S. Chao, et al., Chem. Commun. (Camb.) 54 (2018) 9817-9820; (c) H. Shi, X. Cheng, Q. Lin, et al., Chin. J. Org. Chem. 38 (2018) 1718-1724; (d) Y. Sun, F. Zhang, J. Quan, et al., Nat. Comm. 9 (2018) 2617; (e) J.Y. Chen, W.W. Hao, M. Zhang, et al., Faraday Discuss. 209 (2018) 149-159; (f) J. Wu, J. Tian, L. Rui, W. Zhang, Chem. Commun. (Camb.) 54 (2018) 7629-7632. |
| [9] |
(a) X.S. Du, C.Y. Wang, Q. Jia, et al., Chem. Commun. (Camb.) 53 (2017) 5326-5329; (b) S. Dong, J. Yuan, F. Huang, Chem. Sci. 5 (2014) 247-252; (c) X. Zeng, H. Deng, X. Jia, et al., Chem. Commun. (Camb.) 54 (2018) 11634-11637; (d) Y. Han, C.Y. Nie, S. Jiang, J. Sun, C.G. Yan, Chin. Chem. Lett. 31 (2020) 725-728. |
| [10] |
C.L. Sun, K.X. Teng, L.Y. Niu, Y.Z. Chen, Q.Z. Yang, Acta Chim. Sinica 76 (2018) 779-784. DOI:10.6023/A18070258 |
| [11] |
D. Xia, P. Wei, B. Shi, F. Huang, Chem. Commun. (Camb.) 52 (2016) 513-516. DOI:10.1039/C5CC08038J |
 2020, Vol. 31
2020, Vol. 31 

