b Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, China;
c International Joint Research Center for Sustainable Urban Water System, Tongji University, Shanghai 200092, China
Algae blooms occurring worldwide pose series of challenges to drinking water production and ecology health. Since the mid-1980s, cyanobacteria blooms have occurred every summer in the northern part of Tai Lake, China's third largest freshwater lake [1]. Because of electrostatic repulsion, surface hydrophilicity, and steric effects, algal cells are stable in water and can cause the release of algal organic matter (AOM) [2, 3]. Excessive algal cells and vast released AOM will lead to the problems of algal toxins, taste and odor compounds, and chlorinated disinfection by-products (DBPs) [4-8]. In addition, algal cells are known to be enriched in organic nitrogen including proteins, amino acids, and amines, which contribute to the formation of nitrogenous DBPs (N-DBPs) with genotoxicity and carcinogenicity 2–3 orders of magnitude higher than those of regulated carbonaceous DBPs (C-DBPs) [6, 9].
Many treatment strategies have been investigated to resolve the issue of algae-laden water, such as copper sulfate inhibition [10], air flotation [11], microfiltration [12], ultrafiltration [13], and coagulation-sedimentation-filtration [14]. However, large dosages are required for copper sulfate inhibition and the residual copper would be a threat to aquatic organisms [15]. In addition, low-molecular-weight components of algal metabolites cannot be removed efficiently by air flotation or direct filtration and these methods are usually hindered by large investment and operational cost [15-17]. Traditional coagulation-sedimentation-filtration method is one of the core processes to treat drinking water [14], but the coagulation process using aluminum or ferric salts always shows limited removal efficiency for algae and AOM due to the high mobility, negatively charged surface, and diverse morphology of algal cells [15]. Hence, assistant processes should be developed based on the conventional process to enhance the removal of algal cells and AOM.
It was reported that pre-oxidation is a feasible and popular way to efficiently assist algae removal during the subsequent coagulation-sedimentation process through changing zeta potential, destroying the organic coating and inactivation of viable algae [18, 19]. The effects of commonly used oxidation methods, including chlorine, ozone, permanganate, ferrate, and UV based processes, for enhancing the removal of algae in the following coagulation-sedimentation process have been studied [15, 20-23]. However, some drawbacks of above processes cannot be ignored when they are applied in algae-laden water treatment to aid coagulation. Ferrate has very strong oxidizing ability and its reduction product Fe(III) is nontoxic, but ferrate ion is intrinsically unstable in water and its self-decay behavior is sensitive to the solution environment [24]. UV based oxidation processes always lead to high energy consumption [25]. Moreover, the efficiency of oxidants photolysis highly depends on water quality and the generated reactive radicals may be scavenged by various matrices like ammonia, bicarbonate, and dissolved organic matters [26]. Pre-chlorination of algae-laden water usually results in the generation of chlorinated DBPs occurring with the severe lysis of algal cells [5]. Ozonation can also cause algal cell rupture and release intracellular organic matter (IOM) when applied at relatively high dosages [17]. Besides, it was reported that ozonation of water containing bromide may lead to the formation of bromate and organic brominated by-products which are hazardous to human health [27]. Compared to ozone, permanganate is a relatively mild oxidant and its reduction product MnO2 has high adsorptive and coagulation-aiding capacities [28]. However, only very low inlet permanganate concentration is allowed to avoid cell damage and the appearance of chromaticity in treated water [29].
In our previous studies, bisulfite-activated permanganate technology (PM/BS) was developed as a novel advanced oxidation technology to overcome the disadvantages of traditional permanga-nate pre-oxidation [30-32]. Due to the addition of bisulfite, the appearance of chromaticity arising from permanganate could be avoided. Furthermore, the combination of reactive manganese species (Mn(VI), Mn(V), and possible Mn(III)) and radicals (SO4·- and HO·) derived from PM/BS process contributed to the extremely rapid oxidation of various emerging organic contaminants, including some refractory pollutants resisted to permanganate alone [32-34]. Considering the rapid reaction rates and powerful oxidation potential, PM/BS process might be a promising pre-oxidation technology to mediate the problem of algae blooms.
In this study, PM/BS technology was proposed as a pre-oxidation method to enhance Microcystis aeruginosa (M. aeruginosa) removal by coagulation-sedimentation process. The specific objectives were: (1) To investigate the influence of PM/BS pre-oxidation on the removal efficiency of algal cells and dissolved organic carbon (DOC) by coagulation under various conditions; (2) to analyze the influence of PM/BS pre-oxidation on the changes of algae characteristics and the variation of AOM components; (3) to study the impact of PM/BS pre-oxidation on DBPs concentration formed during the chlorination of settled water; (4) to evaluate the algae removal efficiency by PM/BS process-enhanced coagulation in real algae-laden water.
The details of materials and real water sample used in this study were listed in Text S1 (Supporting information) and all of the analytical methods were supplied in Text S2 (Supporting information).
Fig. 1 shows the influence of PM/BS oxidation on the removal of algal cells and DOC in the subsequent Al2(SO4)3 coagulation process. Without pre-oxidation treatment, the removal rates of algal cells and DOC were as low as 5.4% and 1.0%, respectively, at the Al2(SO4)3 dosage of 30.0 mg/L. When the algae solution was pretreated by PM/BS process with 3.0 mg/L KMnO4 and 10.0 mg/L NaHSO3, the removal rates of algal cells and DOC were raised to 51.2% and 39.6%, respectively, after the coagulation process by using 30.0 mg/L Al2(SO4)3 (Figs. 1a and b). With the increase of Al2(SO4)3 dosage, the removal rates of algal cells and DOC were enhanced in both tests. When the Al2(SO4)3 dosage was increased to 40.0 mg/L, the algae removal rates of the two tests were both over 86.2% and the DOC removal rates were over 47.9%. The results demonstrated that compared to the sole coagulation, PM/BS pretreatment could improve the coagulation efficiency of M. aeruginosa and DOC to a greater extent at a lower dosage of Al2(SO4)3. As displayed in Fig. 1c, it is obvious that the variation trend of zeta potential was in accordance with algae removal performance. The zeta potential of the flocs in PM/BS test was always greater than that in sole coagulation test at different dosage of Al2(SO4)3. It had been reported that the increase in zeta potential of algae cells was a vital reason for pre-oxidation to improve coagulation [18, 23]. The correlation between algae removal and zeta potential in this test was illustrated in Fig. S1 (Supporting information). It was obvious that the lower absolute value of zeta potential was accompanied by the higher algae removal. In this study, the surface of untreated algal cell was highly negatively-charged with a zeta potential of -27.2 mV, which was elevated to -23.8 mV by PM/BS pre-oxidation. As shown in Fig. 1c, PM/BS pre-oxidation combing with Al2(SO4)3 coagulation could increase zeta potential of the flocs by 1.8–4.2 mV compared to that in sole coagulation test, suggesting that PM/BS pretreatment could effectively change the surface properties of M. aeruginosa cells.

|
Download:
|
| Fig. 1. The effect of PM/BS pre-oxidation on the (a) algae removal, (b) DOC removal by Al2(SO4)3 coagulation; (c) Influence of PM/BS pre-oxidation on zeta potential of the flocs after subsequent Al2(SO4)3 coagulation. | |
To further elucidate the impact of PM/BS process on M. aeruginosa, the size distributions of algal cells before and after pre-oxidation were explored. As illustrated in Fig. 2a, the size of raw algal cells mainly fell in the range of 1.5–10.0 μm, which was in agreement with the size of M. aeruginosa cells reported in the literature [23]. After PM/BS pre-oxidation, the size distribution range of M. aeruginosa cells was narrowed with the median particle size (d50) decreasing from 4.03 μm to 3.33 μm. It had been reported that large amount of organic matters were adsorbed on the surface of algal cells and resulted in the expansion of algae size [18]. The change of algae size after PM/BS pre-oxidation might be ascribed to two reasons: (1) PM/BS process brought an acute damage to M. aeruginosa and led to the cell rupture and (2) the adsorbed organic matters on algal cells were oxidized by PM/BS process and separated from the algal cell surface. Thus, the surface morphologies of algal cells and flocs with and without PM/BS pretreatment were investigated by using the SEM to visually investigate the possible impact of PM/BS pretreatment on algal cells and improvement of coagulation performance. Fig. S2a (Supporting information) shows the surface morphology of M. aeruginosa without pretreatment and Fig. S2b (Supporting information) was the SEM micrograph of M. aeruginosa pre-oxidized by PM/BS process. Obviously, the integrity of algal cells was preserved after PM/BS pre-oxidation at the dosages of KMnO4 and NaHSO3 up to 3.0 mg/L and 10.0 mg/L, respectively. Hence, the decrease of algae size after PM/BS pretreatment was on account of the detached organic matter from the surface of algal cell, which could result in the negativity reduce of particle surface as reported [2, 35]. Fig. 2b shows the effect of PM/BS process on floc size growth during the Al2(SO4)3 coagulation of M. aeruginosa-laden water. The flocs rapidly grew in the initial 240 s and reached a plateau for coagulation of M. aeruginosa-laden water without pre-oxidation. For PM/BS process-enhanced coagulation, the floc size in the initial stage was smaller than that in the sole coagulation test and a plateau was reached within 360 s. Then, the floc size increased slowly in the plateau stage and was much larger than that in control sample. As displayed in Figs. S2c and d (Supporting information), compared to the sole coagulation test, PM/BS pre-oxidation could result in the formation of more compact flocs during the post coagulation and enhance the destabilization and aggregation of algae, which could be attributed to the in-situ formed MnO2. It had been reported that MnO2 produced in-situ during KMnO4 pre-oxidation could increase the size of flocs and the collision frequency of particles in water, along with the fact that in-situ formed MnO2 could also be adsorbed on the cell surface through surface bonding to increase the density of flocs [36, 37]. Therefore, for PM/BS process-enhanced coagulation, the smaller floc size in the initial stage in comparison with that in control test was due to the decrease of algae size due to pre-oxidation while the formation of bigger flocs in the plateau stage could be associated with the in-situ formed MnO2 colloids (Fig. 2b).

|
Download:
|
| Fig. 2. The effect of PM/BS pre-oxidation on the (a) size distribution of algal cells and (b) floc size evolution during the coagulation process. | |
The effects of pH and Ca2+ on algae removal with PM/BS pre-oxidation were also investigated. Fig. S3a (Supporting information) shows the removal of M. aeruginosa cells by PM/BS pre-oxidation combing with Al2(SO4)3 coagulation under three pH values, namely 8.0, 8.5, and 9.0, for the water is generally alkaline when algae blooms occur. With the Al2(SO4)3 dosage increasing from 20.0 to 60.0 mg/L, the algae removal rate all increased at the above mentioned three pH levels. In addition, the removal of algal cells was enhanced with the decrease of water alkalinity, especially at the Al2(SO4)3 dosage of 40.0 mg/L. On the one hand, the zeta potential of M. aeruginosa cells could be neutralized by the positively charged hydrogen ions in weaker alkaline condition [19], which was further confirmed by the result that the value of the zeta potential increased from -28.79 mV to -27.24 mV with the solution pH decreasing from 9.0–8.0. On the other hand, the solution pH could also affect the hydrolysis of Al2(SO4)3, which in return influenced the Al2(SO4)3 coagulation of algal cells [38, 39]. It was reported that the total hardness of eutrophic raw water could be up to over 200 mg/L as CaCO3 [20]. Thus, to investigate the effect of water hardness on algae removal by PM/BS process-enhanced coagulation, various amounts of Ca2+ were added into the synthetic algae suspensions so as to have the hardness value varied from 0 to 400.0 mg/L as CaCO3. Fig. S3b (Supporting information) shows that the algae removal was progressively enhanced with the increase of Ca2+ concentration. The enhancement of algae removal by the existence of Ca2+ can be attributed to the increase of surface charge of algal cell. This can be demonstrated by the less negative zeta potential value of the M. aeruginosa when Ca2+ exists, compared to the control sample (Fig. S3c in Supporting information). With the Ca2+ dosage was up to 400.0 mg/L as CaCO3, the zeta potential of algal cells increased to 8.3 mV, while zeta potential of the control sample was -27.2 mV. The correlation between algae removal and zeta potential was shown in Fig. S4 (Supporting information) and the result was consistent with that in Fig. S1. In addition, the surface of in-situ formed MnO2 was also negatively charged and Ca2+ might be served as bridges to hold the two negatively charged surfaces together [20, 36]. Therefore, the existence of Ca2+ could further improve the algae coagulation removal by PM/BS pre-oxidation.
Fig. S5 (Supporting information) shows the effect of KMnO4 dosages ranging from 1.0 mg/L to 7.0 mg/L on algae removal by PM/ BS pretreatment during the subsequent Al2(SO4)3 coagulation-sedimentation. Obviously, the removal rate of M. aeruginosa was enhanced with the increase of initial KMnO4 dosage from 1.0 mg/L to 7.0 mg/L, which could be ascribed to the formation of more MnO2 colloids and active oxidants during PM/BS process.
To further investigate the effect of KMnO4 and NaHSO3 dosages on the dissolved organics in M. aeruginosa solution (without dosing humic acid (HA)), a high-performance size exclusion chromatog-raphy coupled with single-stream multidetectors (SEC-SSMD) was applied for the fluorescence analysis and DOC detection. More details of the method were supplied in Text S2 (Supporting information). As illustrated in Fig. S6a (Supporting information), in raw M. aeruginosa solution, the extracellular AOM contained three major fluorescence peaks, namely peak A (17.3 min, 320 nm), peak B (60.3 min, 394 nm), and peak C (78.7 min, 324 nm), which was consistent with the three-dimensional fluorescence results in previous report [23]. Peaks A and C represented the protein-like components of the extracellular AOM and peak B was related to the humic-like organics [23, 40]. After PM/BS pre-oxidation, the fluorescence intensities of peaks A, B, and C were all decreased and a new fluorescence peak D (57.0 min, 343 nm) appeared, meanwhile, which was also connected to the protein-like materials [23]. With the increase of oxidant dosages, the intensity of peak D was gradually enhanced, which could be due to the AOM secretion from algal cells or the degradation of protein-like organics in peak A, for the molecular weight in peak A was larger than that in peak D. Compared to the raw M. aeruginosa solution, two new peaks (peaks I and V) appeared in the DOC spectra after PM/BS pre-oxidation with the KMnO4 dosage up to 3.0 mg/L. Fig. S7 (Supporting information) summarizes the DOC results so that the concentration change of different molecular weight substances could be observed directly. The organics in peak I has the largest molecular weight in the AOM in M. aeruginosa solution, so the occurrence of peak I should be ascribed to the secretion of macromolecular AOM from algal cells after PM/BS pre-oxidation. Peak II only existed in raw M. aeruginosa solution, suggesting that the materials in peak II were the components of AOM in untreated M. aeruginosa solution and were easily degraded by PM/BS process. Peak III also represented the organics in raw M. aeruginosa solution, but it could be slightly oxidized by PM/BS process with the KMnO4 dosage up to 5.0 mg/L and no significant degradation was observed with the KMnO4 dosage added further. The variation trend of peak V was similar to that of peak I, but the substance in peak V had the minimum molecular weight among all the AOM components in Fig. S7, which indicated that the substance in peak V was derived from the degradation of macromolecular AOM. In addition, it was observed that the intensity of peak IV reached to the maximum at a KMnO4 dosage of 1.0 mg/L or 7.0 mg/L, while the intensity decreased to the minimum at a KMnO4 dosage of 3.0 mg/L or 5.0 mg/L. Oxidants can not only degrade organic compounds but also stimulate algal cells to release AOM, thus, too high or too low concentration of oxidants would both lead to undesirable results. Furthermore, when the KMnO4 dosage increased from 3.0 mg/L to 7.0 mg/L, the contents of organic matters in macromolecular region did not increase obviously, suggesting that the integrity of algal cells did not change significantly. It has been reported that when the KMnO4 dosage was less than 20.0 μmol/L (3.0 mg/L), the integrity of algal cells could be maintained after KMnO4 pre-oxidation [41]. Hence, PM/BS process might keep the integrity of algal cells with a higher KMnO4 dosage in comparison with sole KMnO4 oxidation.
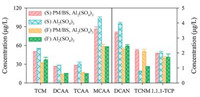
Fig. 3 shows the influence of PM/BS pre-oxidation on DBPs formation during the chlorination of settled water. The dosage of Al2(SO4)3 was chosen to be 40.0 mg/L, for the high removal rate of algal cells with and without PM/BS process at this dosage, so as to investigate whether PM/BS pre-oxidation would bring health risk to drinking water during post chlorination. With PM/BS pre-oxidation, compared to the results in control test, the concentrations of trichloromethane (TCM), chloroacetic acid (MCAA), dichloroacetic acid (DCAA), trichloroacetic acid (TCAA), dichloroacetonitrile (DCAN), and 1, 1, 1-trichloroacetone (1, 1, 1-TCP) were decreased by 8.9%, 16.4%, 8.0%, 10.2%, 15.5% and 2.6%, respectively, while that of trichloronitromethane (TCNM) increased from 0.8 μg/L to 2.1 μg/L. It was noteworthy that trihalomethanes (THMs) and haloacetic acids (HAAs) were the major DBPs generated during the post chlorination, because phycocyanin, substantially existing in AOM, was an important precursor of THMs and HA was the precursor of both THMs and HAAs [23, 42, 43]. The decrease in generated DBPs could be ascribed to the enhanced removal rates of algal cells and DOC with PM/BS pre-oxidation (Fig. 1). It has been reported that nitro-compounds are important precursors of TCNM [44], the increase of TCNM formation might be attributed to the generation of nitro-compounds during the PM/BS pre-oxidation.
|
Download:
|
| Fig. 3. The effect of PM/BS pre-oxidation on DBPs formation during the post chlorination of settled (S) or filtered (F) water. Reaction conditions: [Cl2]0 = 5.0 mg/L | |
Fig. 3 also displayed the effect of PM/BS pre-oxidation on DBPs generated during the chlorination of filtered water after Al2(SO4)3 coagulation. Compared to the results connected with settled water, few algal cells existed in the filtered water and the components were mainly AOM and HA. The obvious decrease of TCM could be due to the degradation of AOM and HA for the oxidation capacity of PM/BS process. With PM/BS pre-oxidation, the concentrations of TCM, DCAA, DCAN, and 1, 1, 1-TCP were dropped by 13.2%, 3.9%, 9.1%, and 4.0%, respectively. Meanwhile, the concentrations of MCAA, TCAA, and TCNM were increased 0.7 μg/L, 1.4 μg/L, and 1.0 μg/L, respectively. The total concentration of generated DBPs in filtered water was much less than that in settled water, suggesting that algal cells in settled water were significant precursors of DBPs. Above all, it can be concluded that the release of organic matters from algal cells caused by PM/BS pre-oxidation did not increase the risk of DBPs detected in this study.
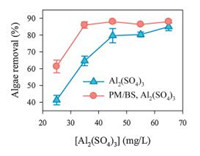
The effect of PM/BS pre-oxidation on algae removal from real algae-laden water after Al2(SO4)3 coagulation was also investigated to evaluate the enhancement of coagulation efficiency by PM/BS process in real algae blooming water. The water parameters of the real algae-laden water were summarized in Table S2 (Supporting information). As illustrated in Fig. 4, after PM/BS pre-oxidation, the algae removal was enhanced from 61.3%–86.0% with the increase of Al2(SO4)3 dosage from 25.0 mg/L to 35.0 mg/L, and reached to a plateau with the further increase of Al2(SO4)3 dosage. However, for sole Al2(SO4)3 coagulation, the removal rate of algal cells was only 64.7% with the Al2(SO4)3 dosage of 35.0 mg/L and reached to 85.0% when the Al2(SO4)3 dosage was up to 65.0 mg/L. The dosage of coagulant could be saved by 46% to obtain 85.0% algae removal when PM/BS pre-treatment was applied. It can be seen that the enhancement of coagulation efficiency for algae removal by PM/BS process in real algae-laden water was even more significant than that in synthetic algae suspensions.
|
Download:
|
| Fig. 4. The effect of PM/BS pre-oxidation on algae removal of real algae-laden water. and the chlorination time was 24 h. Reaction conditions: OD680 = 0.15, pHini = 9.1, [KMnO4]0 = 3.0 mg/L, [NaHSO3]0 = 10.0 mg/L. | |
In this study, the enhancing effect of PM/BS pre-oxidation on M. aeruginosa-laden water treatment by coagulation was investigated. The effects of pH values, Ca2+ concentration, and oxidant dosages on the algae removal and AOM characteristics after Al2(SO4)3 coagulation were evaluated. In addition, the DBPs formed during the chlorination of settled water were detected and the coagulation efficiency of algal cells by PM/BS pre-oxidation was studied in real algae-laden water. Compared to the sole coagulation, PM/BS pretreatment could significantly improve the M. aeruginosa removal with the Al2(SO4)3 dosage of 30.0 mg/L. PM/BS process enhanced the coagulation efficiency because of the increased zeta potential of algal cells after PM/BS pre-treatment and the formation of more compact flocs without destroying the integrity of algal cells. The increase of oxidants dosages could further enhance the removal of algae and meanwhile macromolecular AOM could be degraded into smaller molecular components. The removal of M. aeruginosa cells after PM/BS process-enhanced coagulation was improved as pH dropped from 9.0 to 8.0, because the zeta potential could be neutralized by the positive charged hydrogen ions. Furthermore, after PM/BS pre-oxidation, the existence of Ca2+ could significantly improve the algae coagulation removal, which could be explained by both charge neutralization and bridging between in-situ formed MnO2 and algal cells. PM/BS pretreatment can also reduce the concentration of generated DBPs during the post chlorination of settled water. The coagulation efficiency of algae removal in real algae-laden water was confirmed to be improved by PM/BS process in this study. This study suggested that PM/BS pre-oxidation could effectively improve the coagulation removal of M. aeruginosa and meanwhile control the DBPs formation during the post chlorination.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21976133).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.12.036.
| [1] |
B.Q. Qin, P.Z. Xu, Q.L. Wu, L.C. Luo, Y.L. Zhang, Hydrobiologia 581 (2007) 3-14. DOI:10.1007/s10750-006-0521-5 |
| [2] |
P.C. Xie, J. Ma, J.Y. Fang, et al., Environ. Sci. Technol. 47 (2013) 14051-14061. DOI:10.1021/es4027024 |
| [3] |
S.Q. Zhou, Y.S. Shao, N.Y. Gao, et al., Water Res. 52 (2014) 199-207. |
| [4] |
J.O. Jo, S.D. Kim, H.J. Lee, Y.S. Mok, Chem. Eng. J. 247 (2014) 291-301. DOI:10.1016/j.cej.2014.03.018 |
| [5] |
A. Zamyadi, Y. Fan, R.I. Daly, M. Prevost, Water Res. 47 (2013) 1080-1090. |
| [6] |
J.Y. Fang, J. Ma, X. Yang, C. Shang, Water Res. 44 (2010) 1934-1940. |
| [7] |
B.G. Zhang, S.Q. Zou, R.Q. Cai, M. Li, Z. He, Appl. Catal. B 224 (2018) 383-393. DOI:10.1016/j.apcatb.2017.10.065 |
| [8] |
Q. Lv, B.G. Zhang, X. Xing, et al., J. Hazard. Mater. 347 (2018) 141-149. DOI:10.1016/j.jhazmat.2017.12.070 |
| [9] |
S.D. Richardson, M.J. Plewa, E.D. Wagner, R. Schoeny, D.M. DeMarini, Mutat. Res. 636 (2007) 178-242. DOI:10.1016/j.mrrev.2007.09.001 |
| [10] |
D.M. Mcknight, S.W. Chisholm, D.R.F. Harleman, Environ. Manage. 7 (1983) 311-320. DOI:10.1007/BF01866913 |
| [11] |
A. Zamyadi, S. Dorner, S. Sauve, et al., Water Res. 47 (2013) 2689-2700. |
| [12] |
S. Babel, S. Takizawa, Desalination 261 (2010) 46-51. DOI:10.1016/j.desal.2010.05.038 |
| [13] |
B. Liu, F.S. Qu, H. Liang, et al., J. Membr. Sci. 528 (2017) 178-186. DOI:10.1016/j.memsci.2017.01.032 |
| [14] |
M.A. Shannon, P.W. Bohn, M. Elimelech, et al., Nature 452 (2008) 301-310. DOI:10.1038/nature06599 |
| [15] |
P.L. Jia, Y.P. Zhou, X.F. Zhang, Y. Zhang, R.H. Dai, Water Res. 131 (2018) 122-130. |
| [16] |
W.F.R. Bare, N.B. Jones, E.J. Middlebrooks, J. Water Pollut. Control Fed. 47 (1975) 153-169. |
| [17] |
B. Liu, F.S. Qu, W. Chen, et al., Water Res. 125 (2017) 72-80. |
| [18] |
P.C. Xie, Y.Q. Chen, J. Ma, et al., Chemosphere 155 (2016) 550-563. DOI:10.1016/j.chemosphere.2016.04.003 |
| [19] |
R. Henderson, S.A. Parsons, B. Jefferson, Water Res. 42 (2008) 1827-1845. |
| [20] |
J.J. Chen, H.H. Yeh, I.C. Tseng, Chemosphere 74 (2009) 840-846. DOI:10.1016/j.chemosphere.2008.10.009 |
| [21] |
J. Ma, W. Liu, Water Res. 36 (2002) 871-878. |
| [22] |
M. Ma, R.P. Liu, H.J. Liu, J.H. Qu, W. Jefferson, Sep. Purif. Technol. 86 (2012) 19-25. DOI:10.1016/j.seppur.2011.10.015 |
| [23] |
Y.Q. Chen, P.C. Xie, Z.P. Wang, R. Shang, S.L. Wang, J. Hazard. Mater. 322 (2017) 508-515. DOI:10.1016/j.jhazmat.2016.10.017 |
| [24] |
Z.Y. Zhang, D.Y. Ji, W.T. Mao, et al., Angew. Chem. Int. Ed. 57 (2018) 10949-10953. DOI:10.1002/anie.201805998 |
| [25] |
I.A. Katsoyiannis, S. Canonica, U. von Gunten, Water Res. 45 (2011) 3811-3822. |
| [26] |
J.Y. Fang, Y. Fu, C. Shang, Environ. Sci. Technol. 48 (2014) 1859-1868. DOI:10.1021/es4036094 |
| [27] |
Q.Y. Wu, Y.T. Zhou, W. Li, et al., Water Res. 162 (2019) 43-52. |
| [28] |
J. Naceradska, M. Pivokonsky, L. Pivokonska, et al., Water Res. 114 (2017) 42-49. |
| [29] |
J. Qi, H. Lan, S. Miao, et al., Water Res. 88 (2016) 127-134. |
| [30] |
B. Sun, X. Guan, J. Fang, P.G. Tratnyek, Environ. Sci. Technol. 49 (2015) 12414-12421. DOI:10.1021/acs.est.5b03111 |
| [31] |
B. Sun, H. Dong, D. He, D. Rao, X. Guan, Environ. Sci. Technol. 50 (2016) 1473-1482. DOI:10.1021/acs.est.5b05207 |
| [32] |
B. Sun, D. Li, W. Linghu, X. Guan, Chem. Eng. J. 339 (2018) 144-152. DOI:10.1016/j.cej.2018.01.131 |
| [33] |
B. Sun, Z. Xiao, H. Dong, et al., J. Hazard. Mater. 363 (2019) 412-420. DOI:10.1016/j.jhazmat.2018.10.002 |
| [34] |
J. Chen, D. Rao, H. Dong, et al., J. Hazard. Mater. (2019) 121735.. |
| [35] |
J.Q. Jiang, B. Lloyd, Water Res. 36 (2002) 1397-1408. |
| [36] |
J.J. Chen, H.H. Yeh, Water Res. 39 (2005) 4420-4428. |
| [37] |
J. Ma, N. Graham, G. Li, J. Water Supply Res. Technol. AQUA 46 (1997) 1-10. |
| [38] |
T.T. Guo, Y.L. Yang, R.P. Liu, X. Li, Sep. Purif. Technol. 189 (2017) 279-287. DOI:10.1016/j.seppur.2017.06.066 |
| [39] |
X.X. Ma, Y.A. Wang, S.Q. Feng, S.B. Wang, Environ. Earth Sci. 74 (2015) 3795-3804. DOI:10.1007/s12665-015-4093-4 |
| [40] |
W. Chen, P. Westerhoff, J.A. Leenheer, K. Booksh, Environ. Sci. Technol. 37 (2003) 5701-5710. DOI:10.1021/es034354c |
| [41] |
J. Qi, H.C. Lan, S.Y. Miao, et al., Water Res. 88 (2016) 127-134. |
| [42] |
L. Li, N.Y. Gao, Y. Deng, J.J. Yao, K.J. Zhang, Water Res. 46 (2012) 1233-1240. |
| [43] |
Y. Mao, D. Guo, W. Yao, et al., Water Res. 130 (2018) 322-332. |
| [44] |
A.D. Shah, W.A. Mitch, Environ. Sci. Technol. 46 (2012) 119-131. DOI:10.1021/es203312s |
 2020, Vol. 31
2020, Vol. 31 

