Utilizing solar energy via semiconductor photocatalysis technology has been extensively considered as one of the most promising strategies to address the increasing environmental issues in recent years [1-4]. Copper tungstate (CuWO4), as a typical n-type oxide semiconductor with a bandgap of 2.2 eV, has been widely investigated in the fields of photocatalysts, sensors, and photoelectrochemical (PEC) water splitting owing to its favorable light absorbance in a broad-spectrum range [5-7]. However, limited by the inherently indirect bandgap, the photocatalytic/PEC performance of bare CuWO4 nanostructure is generally hindered by the low production of photo-generated carriers, poor charge carrier mobility, and rapid charge recombination [8-10]. Therefore, designing a multi-phase structure to promote carrier density and achieve a swift electron migration would significantly improve the performance of CuWO4. Recently, although various CuWO4 based composites, such as Ag/CuWO4/BiOI [11], Pt/CuWO4/Co3O4 [12] and CuWO4/Mn3O4 [13], have been reported to accelerate electron transfer by forming p-n junction, the noble metals are inevitably used and their potential utilizations are limited to the photoanode in PEC water splitting. Thus, the exploration of CuWO4-based photocatalysts needs to be strengthened urgently.
Fortunately, quantum dots (QDs) sensitization emerges as one of the effective strategies to improve the photovoltaic or photocatalytic efficiency. The excited state electrons of QDs can inject into the conduction band (CB) of adjacent semiconductors in the order of picoseconds, while their instinct binding process with holes in valence band (VB) is in the order of nanoseconds, laying the kinetic foundation of QDs-sensitized charge regulation [14, 15]. Specifically, CdS QDs, a popular sensitizer with excellent photoelectric properties originating from quantum confinement effects, shows aligned CB and VB positions favoring the formation of type-II heterojunction with CuWO4 [16-18]. It is well known that the built-in potential gradient at the heterostructure interface can regulate and accelerate the electron migration to the active sites [19-21]. Furthermore, carbon dots (CDs), as a newly developed carbon nanomaterial with merits of non-toxicity, high chemical stability, and earth abundance, have been deemed as the benign electron reservoir and conductor on account of its superior electron transfer ability, thus can serve as the superfast charge tunnels in optoelectronic devices [22-24].
Accordingly, modification of CuWO4/CdS surface with CDs will generate effective charge transfer tunnels and accelerate the electron transfer from the semiconductor to the organic pollutants, thereby enhance the photocatalytic degradation activity. Herein, we rationally designed and synthesized a ternary CuWO4/CdS/CDs hollow sphere heterostructure. Under the synergistic sensitization of CdS QDs and CDs to CuWO4 hollow spheres, a cascaded electron transfer pathway from CdS to CuWO4 and then to the organics through CDs tunnels is realized, and the degradation performance towards organic pollutants such as phenol and congo red (CR) is enhanced. The electron transfer kinetics process and the photocatalytic mechanism of the as-obtained ternary composite are also discussed in detail.
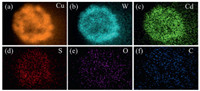
Fig. 1a briefly illustrates the synthetic route of CuWO4/CdS/CDs (CuWSC). Different amounts of carbon dots modified CuWO4/CdS (CuWS) catalysts were named CuWSC1, CuWSC2 and CuWSC3 respectively. The crystal structure of the as-prepared samples was characterized by X-Ray diffraction (XRD) measurement. All the diffraction peaks can be assigned to triclinic CuWO4 phase (JCPDS No. 72-0616), in which the typical (010), (100), (1 0 1), (1 1 1) and (111) crystal faces of CuWO4 are readily indexed (Fig. S1 in Supporting information). It should be noted that no diffraction peaks of CdS and CDs are observed due to the low content. The successful formation of CDs can be demonstrated by transmission electron microscopy (TEM) image and bright green fluorescence (Fig. S2 in Supporting information). The hollow sphere morphologies can be clearly observed for all the as-obtained samples, even after decorating CdS and CDs, the hollow sphere morphology can be well maintained (Fig. 1b and Fig. S3 in Supporting information). The average diameter of the CuWSC2 spheres is ~ 400 nm and the shell thickness is ~50 nm. The unique hollow sphere morphology can not only shorten the electron transport path and accelerate the charge carrier separation, but also increase the utilization of light owing to its multiple refractions of the absorbed light by the inner cavity [25]. Therefore, the as-prepared CuWO4 hollow spheres can serve as an ideal skeleton for a good photocatalyst. From the high resolution transmission electron microscopy (HRTEM) image, the large area with the crystal plane spacing of 0.31 nm, which corresponds exactly to the (1 1 1) crystal plane of CuWO4 crystal, can be assigned to be the main matrix CuWO4 (Figs. 1c and d). The aggregated quantum dots with the diameter less than 5 nm can be designated as CdS (Fig. 1e) and CDs (Fig. 1f), respectively, with a spacing value of 0.35 nm corresponding to the (111) plane of CdS and another spacing value of 0.28 nm corresponding to CDs [26, 27]. Moreover, the energy-dispersive spectroscopy (EDS) elemental mapping images and the spectrogram demonstrate that the Cu, W, Cd, S, O and C elements are uniformly distributed in the hollow sphere (Figs. 2a–f and Fig. S4 in Supporting information). X-ray photoelectron spectroscopy (XPS) spectra show that the CuWSC2 sample contains Cu, W, O, Cd, S and C elements, consistent with the TEM mapping results, further confirming the successful hybridization and formation of the ternary composites (Figs. S5-S7 Supporting information). All the results indicate that the ternary heterostructure has been constructed.

|
Download:
|
| Fig. 1. (a) Schematic diagram for the synthesis of CuWO4/CdS/CDs (CuWSC). (b) TEM, (c–f) HRTEM images of the CuWSC2 composite. | |
|
Download:
|
| Fig. 2. (a–f) EDS mapping images of the CuWSC2 composite. | |
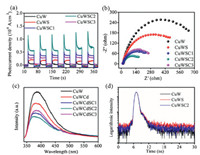
The light response in UV–vis range of the ternary composite is enhanced after the decoration of CdS QDs and CDs (Fig. S8 in Supporting information). It is well known that efficient charge separation/transfer is crucial for the improvement of photocatalytic performance. Notably, the photocurrent density of the ternary catalyst CuWSC2 is much higher than those of the binary CuWS and the single CuW (Fig. 3a), which indicates the higher charge separation rate in the ternary CuWSC2. The enhanced charge separation in CuWSC can be ascribed to the good photoelectric performance of CdS QDs as well as the excellent electron conductivity of CDs. Moreover, the CuWSC2 shows the smallest semicircle and lowest interfacial charge transfer resistance among all the prepared photocatalysts (Fig. 3b), implying that the modification of CuWO4 with CdS QDs and CDs can significantly increase the interfacial charge mobility. Basically, the intrinsic band-edge photoluminescence (PL) of a semiconductor mainly rises from the recombination of photogenerated electrons and holes. Thus, the weaker the PL intensity of the prepared catalyst, the higher the separation efficiency of photo-generated electron-hole pairs [28, 29]. When CuWO4 was modified with CdS QDs and CDs, the PL intensity decreases to the lowest level at the composition of CuWSC2, and then rises with the increase of CDs amount (Fig. 3c). On basis of the above experiments, it is believed that the modification of CdS QDs and appropriate amounts of CDs onto CuWO4 main matrix with synergetic effects can suppress the recombination of the electron-hole pairs and thus promote the photocatalytic activities.
|
Download:
|
| Fig. 3. (a) Photocurrent responses under simulated solar light irradiation. (b) EIS spectra recorded at 1.67 V vs. RHE. (c) PL spectra and (d) time-dependent PL decay profiles of the prepared samples. | |
The time-dependent PL decaycurves could further untangle the charge dynamic process in the hybridization system. The PL decay was monitored at CuWO4's characteristic emission peak of 390 nm under 320 nm excitation (Fig. 3d). According to the decay lifetime biexponential function (Eq.1) [30, 31], the PL decay process can be well fitted, and the results are shown in Table 1.
|
|
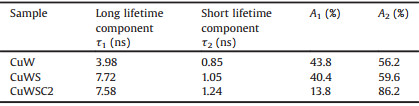
Table 1 The kinetic parameters of PL decay for different samples. |
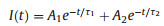
|
(1) |
All the samples exhibit both a long and a short decay lifetime: The long one (τ1) is designated to the inter-band luminescence process and represents lifetime of the excited electrons in CB to recombine with the holes in VB, while the short one (τ2) is associate with the nonradiative transition and represents lifetime of the excited electrons to recombine with the surface defects. The value of A1 and A2 presents the weighted contributions of the two kinds of transition, respectively. Notably, the long lifetime is extended significantly after modification of CdS QDs (τ1 = 7.72 ns) compared to bare CuWO4 (τ1 = 3.98 ns), which indicates that CuWO4 acts as the electron acceptor and CdS serves as the donor on the adjacent interfaces [32-34]. After the further decoration of CDs, the long lifetime of the ternary material CuWSC2 (τ1 = 7.58 ns) only decreases slightly compared to that of the binary CuWS sample (τ1 = 7.72 ns). However, the value of A1 (13.8%) decreases sharply compared to those of the bare CuWO4 (43.8%) and binary CuWS (40.4%) samples, suggesting that the recombination of the carriers has been indeed suppressed by CDs.
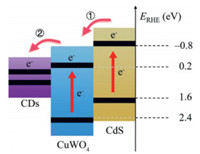
It seems like the electrons are separated and "captured" by the closely contacted CDs with excellent electron storage ability. Once there are appropriate organic pollutants, the CDs will act as transfer tunnels and release the electrons quickly to involve the redoxreaction. Theproposedcascaded electron transitionpathway is illustrated in Fig. 4. The main processes include: 1) Electrons were injected into the CB of CuWO4 from CdS through type-II interface heterojunction; 2) Electrons were conducted from CB of CuWO4 to the surface through CDs, and further reacts with the adsorbed O2 or the organics. All the above results elucidate that CdS and CDs can synergistically accelerate carrier separation and form a cascaded electron transition pathway to enhance a smooth electron migration. The enhanced photocatalytic performance can be expected and achieved.
|
Download:
|
| Fig. 4. Schematic illustration of the electron transfer pathway in ternary CuWSC composite. | |
In order to demonstrate the photocatalytic activities of the prepared photocatalysts and verify the rationality of the above discussion, the degradation of phenol and CR were carried out. The reaction kinetic equation in the degradation process is listed as follows (Eq. 2):

|
(2) |
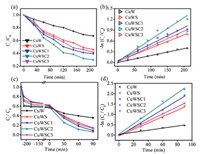
where C0 and Ct represent the initial concentration and the concentration after a certain reaction time, respectively; k stands for the kinetic rate constant; t is the reaction time. Figs. 5a and b show the degradation process of phenol with the prepared catalysts under full spectra illumination. The photocatalytic efficiency of the CuW catalyst only reaches 32.75% after 210 min, while the ternary CuWSC2 accounts for 70.13% efficiency, about 114% enhancement compared to bare CuW. Moreover, the kinetic rate constant of the CuWSC2 (0.00459 min-1) is nearly 2.5-fold higher than that of pure CuWO4 (0.00191 min-1) and is 1.3 times of the binary CuWS (0.00364 min-1). Otherwise, the as-prepared catalysts show similar photocatalytic degradation tendency towards CR (Figs. 5c and d). The photocatalytic efficiency of pure CuWO4 catalyst is only 64.24% after 90 min, and the kinetic rate is only 0.00504 min-1. However, the photocatalytic degradation efficiency of CuWS and CuWSC2 reaches up to 74.62% and 93.66% with the corresponding kinetic rate constant of 0.01554 min-1 and 0.0248 min-1. Compared with bare CuW, the ternarycomposite improves the degradation efficiency towards CR by 46%, and the kinetic rate constant by nearly four times. The degradation efficiency shows no significant decline compared with the initial value, both for phenol and CR (Figs. S9 and S10 in Supporting information). Therefore, it was proved that the ternary hybrid catalyst CuWSC2 displays improved photocatalytic performance than both the single and binary compounds. The photocatalytic experiment results match well with the above conjecture about unidirectional electron migration, and the synergistic effect of CdS QDs and CDs can be demonstrated thoroughly. The modification of excessive amounts of CDs leads to lower reaction kinetics rate, because excessive CDs could aggregate seriously, block the pores and hinder the mass transfer. Based on the above analysis and discussion, the possible photocatalytic mechanism for organic pollutant degradation over the ternary system is illustrated (Fig. S11 in Supporting information). The CB and VB positions of CuWO4 and CdS are aligned to form type-Ⅱ heterojunction, so the excited electrons in CB of CdS spontaneously transfer to CB of CuWO4 and the holes in VB of CuWO4 would transfer to VB of CdS. Afterward, the decorated CDs could collect and transfer electrons due to their excellent electron storage and conductivity and then improve the electron's mobility from CB of CuWO4 to reach surface, finally realizing the unidirectional electrons migration and enhanced photocatalytic activities. The separated electrons/holes could react directly with the organics or react with the adsorbed O2 or H2O to form ·O2-/·OH and then degrade the organic pollutants.
|
Download:
|
| Fig. 5. Photocatalytic degradation of (a) phenol and (c) CR under simulated solar light irradiation; the first-order kinetics curves of (b) phenol and (d) CR degradation. | |
In summary, the ternary CuWO4/CdS/CDs nanospheres with hollow structure were prepared successfully and the photocatalytic degradation performances towards phenol and CR are enhanced by regulating the electron transfer pathway. Compared with the bare CuWO4 sample, the as-synthesized ternary heterostructure improves the degradation efficiency of phenol and CR by 100% and 46% under solar light illumination respectively, which strongly demonstrates that the surface modification of CdS QDs and CDs can facilitate electron migration within CuWO4, thus effectively maximize the utilization of photo-generated electrons and enhance the photocatalytic degradation activities. These findings highlight the superiorities of synergistic sensitization effect between CdS QDs and CDs and open up new avenues for designing novel CuWO4 based photocatalysts with potential applications in solar energy harvesting and utilizing.
Declaration of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21875048), Guangdong Natural Science Foundation (No. 2017A030313255), Major Scientific Project of Guangdong University (No. 2017KZDXM059), Yangcheng Scholars Research Project of Guangzhou (No. 201831820), Science and Technology Research Project of Guangzhou (No. 201804010047), Guangzhou University's 2017 Training Program for Young TopNotch Personnel (No. BJ201704).
Appendix A. Supplementary dataSupplementary materialrelated tothis article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.12.013.
| [1] |
Y. Zhao, Z. Li, M. Li, et al., Adv. Mater. 30 (2018) 1803127. DOI:10.1002/adma.201803127 |
| [2] |
M. Zhu, Z. Sun, M. Fujitsuka, T. Majima, Angew. Chem. Int. Ed. 57 (2018) 2160-2164. DOI:10.1002/anie.201711357 |
| [3] |
J. Feng, F. Yang, Y. Ye, et al., Nanoscale 11 (2019) 19512-19519. DOI:10.1039/C9NR05453G |
| [4] |
X. Cai, L. Mao, S. Yang, K. Han, J. Zhang, ACS Energy Lett. 3 (2018) 932-939. DOI:10.1021/acsenergylett.8b00126 |
| [5] |
Z. Lin, W. Li, G. Yang, Appl. Catal. B 227 (2018) 35-43. DOI:10.1016/j.apcatb.2018.01.021 |
| [6] |
F. Duanmu, Z. Shen, Q. Liu, S. Zhong, H. Ji, Chin. Chem. Lett. 31 (2020) 1114-1118. DOI:10.1016/j.cclet.2019.07.032 |
| [7] |
Y. Gao, T.W. Hamann, J. Phys. Chem. Lett. 8 (2017) 2700-2704. DOI:10.1021/acs.jpclett.7b00664 |
| [8] |
W. Ye, F. Chen, F. Zhao, N. Han, Y. Li, ACS Appl. Mater. Interfaces 8 (2016) 9211-9217. DOI:10.1021/acsami.6b03176 |
| [9] |
C.M. Tian, M. Jiang, D. Tang, et al., J. Mater. Chem. A 7 (2019) 11895-11907. DOI:10.1039/C8TA12070F |
| [10] |
W. Ding, X. Wu, Q. Lu, Mater. Lett. 253 (2019) 323-326. DOI:10.1016/j.matlet.2019.06.109 |
| [11] |
M. Zhou, Z. Guo, Q. Song, X. Li, Z. Liu, Chem. Eng. J. 370 (2019) 218-227. DOI:10.1016/j.cej.2019.03.193 |
| [12] |
Z. Liu, Q. Song, M. Zhou, et al., Chem. Eng. J. 374 (2019) 554-563. DOI:10.1016/j.cej.2019.05.191 |
| [13] |
K. Li, C. Zhang, X. Li, et al., Catal. Today 335 (2019) 173-179. DOI:10.1016/j.cattod.2018.11.003 |
| [14] |
J.B. Baxter, C. Richter, C.A. Schmuttenmaer, Annu. Rev. Phys. Chem. 65 (2014) 423-447. DOI:10.1146/annurev-physchem-040513-103742 |
| [15] |
K. Wu, Q. Li, Y. Jia, et al., ACS Nano 9 (2015) 961-968. DOI:10.1021/nn506796m |
| [16] |
M. Xing, B. Qiu, M. Du, et al., Adv. Funct. Mater. 27 (2017) 1702624. DOI:10.1002/adfm.201702624 |
| [17] |
M.I. Aziz, F. Mughal, H.M. Naeem, et al., Mater. Chem. Phys. 229 (2019) 508-513. DOI:10.1016/j.matchemphys.2019.03.042 |
| [18] |
M. Zhou, Z. Liu, X. Li, Z. Liu, Ind. Eng. Chem. Res. 57 (2018) 6210-6217. DOI:10.1021/acs.iecr.8b00358 |
| [19] |
B.F. Zheng, T. Ouyang, Z. Wang, et al., Chem. Commun. 54 (2018) 9583-9586. DOI:10.1039/C8CC04199G |
| [20] |
R.B. Wei, P.Y. Kuang, H. Cheng, et al., ACS Sustain. Chem. Eng. 5 (2017) 4249-4257. DOI:10.1021/acssuschemeng.7b00242 |
| [21] |
C. Li, S. Dong, R. Tang, et al., Energy Environ. Sci. 11 (2018) 3201-3211. DOI:10.1039/C8EE01046C |
| [22] |
R.B. Wei, Z.L. Huang, G.H. Gu, et al., Appl. Catal. B 231 (2018) 101-107. DOI:10.1016/j.apcatb.2018.03.014 |
| [23] |
J.F. Li, C.Y. Zhong, J.R. Huang, et al., J. Colloid Interface Sci. 553 (2019) 758-767. DOI:10.1016/j.jcis.2019.06.077 |
| [24] |
Q.K. Chen, L. Chen, J.J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [25] |
X. Liu, M. Ye, S. Zhang, et al., J. Mater. Chem. A 6 (2018) 24245-24255. DOI:10.1039/C8TA09661A |
| [26] |
Y. Zhang, J. He, Phys. Chem. Chem. Phys. 17 (2015) 20154-20159. DOI:10.1039/C5CP03498A |
| [27] |
X.L. Yin, L.L. Li, W.J. Jiang, et al., ACS Appl. Mater. Interfaces 8 (2016) 15258-15266. DOI:10.1021/acsami.6b02687 |
| [28] |
H. Zhao, Z. Hu, J. Liu, et al., Nano Energy 47 (2018) 266-274. DOI:10.1016/j.nanoen.2018.02.052 |
| [29] |
N.C. Zheng, T. Ouyang, Y. Chen, et al., Catal. Sci. Technol. 9 (2019) 1357-1364. DOI:10.1039/C8CY02206B |
| [30] |
J. Ran, T.Y. Ma, G. Gao, et al., Energy Environ. Sci. 8 (2015) 3708-3717. DOI:10.1039/C5EE02650D |
| [31] |
J. Li, Z. Zhang, X. Li, et al., J. Mater. Chem. C 5 (2017) 6294-6299. DOI:10.1039/C7TC01285C |
| [32] |
X. Li, S. Liu, K. Fan, et al., Adv. Energy Mater. 8 (2018) 1800101. DOI:10.1002/aenm.201800101 |
| [33] |
J. Ran, B. Zhu, S.Z. Qiao, Angew. Chem. Int. Ed. 56 (2017) 10373-10377. DOI:10.1002/anie.201703827 |
| [34] |
X. Zhang, Z. Zhu, Z. Guo, Z. Sun, Y. Chen, Chem. Eng. J. 356 (2019) 413-422. DOI:10.1016/j.cej.2018.09.075 |
 2020, Vol. 31
2020, Vol. 31