b College of Chemical Engineering Nanjing Forestry University, Nanjing 210037, China;
c College of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou 310014, China;
d School of Pharmaceutical Sciences, Guangdong Provincial Key Laboratory of New Drug Screening, Southern Medical University, Guangzhou 510515, China;
e State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, China
Bacterial contamination has drawn great research concern in the past decades, as the significant mortality and morbidity worldwide [1]. E. coli, one of the classic gram-negative bacteria, are common contaminants in retail poultry and involved inflammatory bowel disease, urinary tract infections and meningitis in both animals and humans [2]. Therefore, it is important to quickly and sensitively detect the amount of E. coli in environmental and biological samples. Recently, several methods have been developed for the E. coli determining, such as enzyme linked immunosorbent assay (ELISA), magnetic activated cell sorting (MACS), polymerase chain reaction (PCR) and surface plasmon resonance (SPR) [3-6]. However, these current available methods for detecting bacteria require expensive apparatus and sophisticated sample preparation procedures, which limit their broader application.
Quantum dots (QDs), approximately 2–100 nm in diameter, have been at the center of much research in recent years due to their unique optical, magnetic, and electronic properties [7-13]. Unlike conventional fluorescent dyes, QDs have high fluorescence quantum yields, size-tunable photoluminescence spectra, broad absorption, narrow emission wavelength, high photobleaching threshold and excellent photostability, which allow QDs to be used for continuous or chronical monitoring in biological imaging and analysis. Moreover, QDs could show little toxicity in vitro and in vivo with in controlling concentration for meeting the requirement of practical biological processes [14, 15]. Particularly, water-soluble and biological-compatible QDs have played an important role in the photoluminescence response of microorganisms (such as Escherichia coli, Staphylococcus aureus and candida albicans), due to the enhancement of fluorescence intensity and decreasing the requirements for fluorescence detection instrumentations with hyperchromic conjugation agent [16, 17]. Therefore, these unique optical properties of QDs make them to be the excellent candidates for the development of sensitive sensors in microbe monitoring.
To date, there are a few studies in which the usage of QDs for determination of E. coli is reported. Weng et al. reported the effective fluorescent probes for selective labeling E. coli in various matrices using the carbon QDs [18]. Chen et al. successfully developed peptide-based ZnO QDs for E. coli diagnosis and treatment in vivo, which could discriminate the E. coli infection from sterile inflammation or cancer in vivo with high specificity and low detection limitation [19]. Dogan et al. prepared core-shell magnetic QDs (Fe3O4@Au) modified with biotinylated antibodies to detect E. coli sample in real matrices with high selectivity [20]. However, these QDs are usually synthesized with a fairly long reaction time, high temperature and complicated process by a traditional organometallic approach, which led to high cost. Compared with these QDs, zinc telluride (ZnTe) quantum dots can be directlyand simply prepared through a one-pot synthetic route, and the synthesis is more reproducible and inexpensive [21, 22]. Therefore, they have great potential to be one of the most important fluorescent probes in application of biotechnology and medicine.
In the current study, mannose (MAN) modified ZnTe (MAN-ZnTe) QDs were prepared by hydrothermal method, which were proved by transmission electron microscopy (TEM), X-ray diffraction (XRD) and Fourier transform infrared spectrograph (FTIR) techniques. Secondly, the modified QDs using as fluorescence probes for the sensitive determination of E. coli in the presence of other bacteria was studied. Thirdly, the effects of concentration, temperature, pH value and incubation timeon the fluorescence intensity of MAN-ZnTe QDs for the E. coli sensing probes were investigated. Finally, the detection limit of E. coli and the quenching mechanism by MAN-ZnTe QDs were also discussed.
The basic MAN-ZnTe QDs were prepared as follows. First, 50 mL of a 5.0 ×10-3 mol/L MAN solution was mixed with 50 mL 2.5 ×10-2 mol/L zinc acetate solution in a 100 mL three-necked round-bottomed flask. Then the pH was adjusted to 10.0 using 0.1 mol/L NaOH solution. Under vigorous stirring, 25.0 mL of 5.0 ×10-2 mol/L Na2TeO3 solution was dropped slowly into the flask to afford a Se/Zn molar ratio of 1:1. Subsequently, the mixed solution was stirred for 10 h. Finally, the mixed solution was sealed and incubated in an autoclave for 12 h at 100 ℃. After cooling, the ZnTe particles were precipitated out from solution using excess acetone, and the solutions were centrifuged at 5000 rpm for 10 min to harvest the MAN-ZnTe QDs.
The E. coli cells were diluted to a known concentration (i.e., 5.0 ×107CFU/mL) and subsequently incubated with MAN-ZnTe QDs (7.48 × 10-3 g/L) for 1 h under gentleshaking at roomtemperature. The mixtures were centrifuged (4000 rpm, 20 min) and washed twice with phosphate buffer saline (PBS, 0.01 mol/L, pH 7.4). The bacterial pellets were then resuspended in PBS, and transferred separately into 96-well microtiter plates. Finally, the mixture was immediately detected using luminescence spectrometer, and fluorescence spectrawere recorded using an excitation wavelength at 365 nm.
Fig. 1 describes the X-ray diffraction patterns of the MAN-ZnTe QDs. The characteristic diffraction peaks at (2θ) 25.34°, 29.38°, 36.19°, 41.25°, 49.62° and 65.86° could be index to the typical (111), (200), (102), (220), (311) and (331) crystal plane of pure ZnTe, respectively (JCPDS card No. 80-0022) [23-28]. The average size (D) of ZnTe QDs can be calculated according to Scherrer's equation: D = k(λ/βcosθ), where k is a constant equal to 0.89, λ is the X-ray wavelength equal to 0.154 nm, β is the full width at half maximum and θ is the half diffraction angle (25.34°). The calculated result indicated that the average size (D) is 12 nm approximately.

|
Download:
|
| Fig. 1. XRD spectrum of MAN-ZnTe QDs. | |
The TEM image and crystal phase of synthesized ZnTe QDs were investigated (Fig. S1 in Supporting information). It was found that the average diameter of synthesized QDs is about 11 nm. Thus, the size of the nano-sized ZnTe QDs as determined by TEM is in good agreement with the size as calculated from X-ray diffraction by Scherrer's equation.
The FTIR spectra of MAN and MAN-ZnTe QDs were depicted (Fig. S2 in Supporting information). As shown in Fig. S2a, the bands at 3409 cm-1, 1685 cm-1, 1164 cm-1, 873 cm-1 and 804 cm-1 were checked in the FTIR spectrum of MAN. The broad peak at 3409 cm-1 belong to the stretching vibration of O—H bond of MAN; the peaks at 1685 cm-1 was assigned to the stretching vibration of carboxyl gruop; the peak at 1164 cm-1 was generated from the stretching vibration of C—O in glycoside ring; the peak at 873 cm-1 and 804 cm-1 was ascribe to typical stretching vibration of glycoside ring in MAN [29]. After MAN conjugated into ZnTe QDs, such typical absorption peaks of MAN are also detected (Fig. S2b), which suggested that MAN was successfully anchored on ZnTe QDs surface.
The ultraviolet-visible absorption spectrum of MAN-ZnTe QDs was shown (Fig. S3 in Supporting information). It was found that MAN-ZnTe QDs showed a sharp maximum absorption at 214 nm.
The effect of bacteria on fluorescence intensity of MAN-ZnTe QDs was studied (Fig. 2). It was seen that the luminescence intensity of ZnTe QDs was strongly quenched by E. coli in the PBS buffer solution. As for other bacteira (such as S. aureus, B. subtilis and P. aeruginosa), no obvious change in fluorescence intensity was observed in the presence of these bacteria, even at concentrations 10 times greater than that of E. coli. These data indicated that MAN-ZnTe QDs could be used as selective E. coli detectors.

|
Download:
|
| Fig. 2. The effect of bacteria on the fluorescence intensity of MAN-ZnTe QDs. Conditions: concentration of MAN-ZnTe QDs: 7.48 × 10-3 g/L; concentration of E. coli: 5.0 ×107 CFU/mL; for other bacteria: 5.0 ×108 CFU/mL, pH: 7.0. | |
The influence of E. coli concentration on the fluorescence intensity of MAN-ZnTe QDs was examined (Fig. 3). The fluorescence spectrum of synthesized ZnTe QDs was symmetric and narrow with an emission maximum at 563 nm, which was in accordancewith prior reports [21]. Moreover, as shown in Figs. 3b-g, the emission fluorescence intensity of ZnTe QDs was found to decrease dramatically with increasing dosage of E. coli, whereas the maximum emission wavelength and line width almost kept unchanged. The quenching effect of E. coli on the fluorescence emission of MAN-ZnTe QDs was found to be dosage dependent, confirming that MAN-ZnTe QDs could be used as a fluorescence nanosensor for E. coli.

|
Download:
|
| Fig. 3. The effect of E. coli concentrations on the fluorescence intensity of ZnTe QDs. a = 0.0 × 106 CFU/mL; b = 1.0 × 106 CFU/mL; c = 5.0 × 106 CFU/mL; d = 1.0 × 107 CFU/mL; e = 2.0 × 107 CFU/mL; f = 4.0 × 107 CFU/mL; g = 5.0 × 107 CFU/mL; concentration of MAN-ZnTe QDs is 7.48 × 10-3 g/L. | |
Since the property of QDs was strongly dependent upon the temperature to affect the measurements accuracy and sensitivity, the influence of temperature on the fluorescence intensity of MANZnTe QDs in the presence of E. coli was investigated (Fig. S4 in Supporting information). It was seen that the fluorescence intensity of MAN-ZnTe QDs dropped about 34.1% with the temperature varying from 25 ℃ to 55 ℃, which was probably caused by the thermal activation of surface traps leading to nonradiative recombination of excitons [30]. Thus, a room temperature (25 ℃) was selected for all experimental measurements.
In general, pH was one of the major factors that affect the fluorescence of QDs [31]. Therefore, the effect of pH on the fluorescence intensity of MAN-ZnTe QDs-E. coli solution system was shown (Fig. S5 in Supporting information). With increasing pH value from 4.0–7.0, about 2-fold enhancement of the fluorescence was observed, meanwhile, further pH increase (up to 8.0) resulted in slight drop in light emission. The low fluorescence intensity under acidic conditions likely resulted from dissociation of the MAN modified ZnTe QDs by protonation of the surface-binding alcoholates [32]. At pH increased, the deprotonation of the alcoholic hydroxyl group in the MAN molecule was favored, which would strengthen the covalent bond between Zn and MAN molecule, and likely increase the fluorescence intensity. At pH values higher than 7.0, the fluorescence intensity decreased owing to precipitation of Zn(OH)2. Thus, pH of 7.0 appeared to be optimal for the determination of E. coli.
The effect of incubation time on the fluorescence intensity of MAN-ZnTe QDs was showed (Fig. S6 in Supporting information). It was found that the reaction was completed within 20 min at room temperature, and the fluorescence intensity remained unchanged till 90 min. Therefore, the experiments data should be recorded within 20 min–90 min.
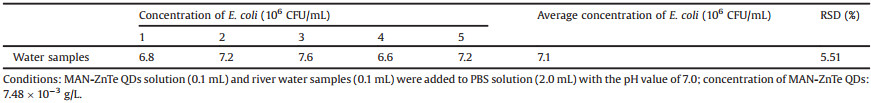
To examine the feasibility of using MAN-ZnTe QDs in practical applications, the detection of E. coli in samples of real-world river water was evaluated (Table 1). Five samples of the river water were collected from the Songshan Lake (Guangdong province, China) near our campus. Prior to testing, the collected river water was filtered by a polypropylene filter membrane. The standard curve of the determination of E. coli was obtained with the concentration range of 1.0 × 105 CFU/mL to 1.0 × 108 CFU/mL, and the quenching MAN-ZnTe QDs by E. coli fitted the linear equation: F0/F = 0.4053 × log[Q] - 1.0815 (the linear regression coefficient (R) is 0.9911). It was found that the average concentration of E. coli in river water was 7.2 × 106 CFU/mL, which was in accordance with an agar plate count method (tested results was 8.0 × 106 CFU/mL). This result indicated that the proposed QDs probe had great potential for use in the determination of E. coli in real samples.
|
|
Table 1 Determination results of E. coli in water samples. |
It was known that the quenching mechanism of QDs was due to static or dynamic interaction of the luminescent molecule and the quencher. Generally, the quenching rate constants increased with increasing temperature in a dynamic quenching mechanism, but the reverse effect was observed in case of static quenching [33]. As the fluorescence intensity of MAN-ZnTe QDs is significantly quenched by the addition of E. coli (Fig. 2), the fluorescence quenching data were analyzed by the quenching equation.
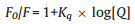
|
Where F0 and F are the fluorescence intensities of QDs in the absence and presence of the quencher, respectively. Kq is the quenching constant and [Q] is the concentration of E. coli.
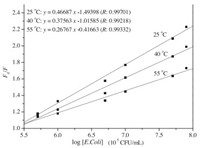
Fig. 4 illustrates the relationship between fluorescence intensity of QDs and concentration of the E. coli at different temperatures. As shown in Fig. 4, linear plots of F0/F versus log[Q] at different temperatures were obtained, and the quenching efficiency of ZnTe QDs decreased with the temperature increasing. These results implied that the quenching was not collided by the dynamic collision, but initiated by the static quenching [34]. Moreover, the limit of detection was evaluated using 3σ/S, and was found to be 4.6 × 104 CFU/mL, where σ is the standard deviation of the blank signal from 10 samples, and S is the slope of the linear calibration plot at room temperature. The limit of detection here was better than that of plate counting method [35]. The recoveries in the real samples were between 99%–104%, and the RSD value was 2.59%. This result demonstrated that the ZnTe QDs was reliable and practical.
|
Download:
|
| Fig. 4. Fluorescence quenching of MAN-ZnTe QDs with E. coli at different temperatures. | |
In summary, MAN modified ZnTe QDs obtained by hydrothermal synthesis were used to construct a fluorescence sensor for E. coli detection. The results showed that MAN-ZnTe QDs exhibited good selectivity toward E. coli, and the detection limit was 4.6 × 104 CFU/mL at pH 7.0, 25 ℃, 20 min incubation time. The probable quenching mechanism might be a static quenching procedure, and the obtained probes were successfully used to detect E. coli in lake water samples, showing perspectives of environmental determination for future research. And the hydrothermal synthesis of functional MAN-ZnTe QDs developed in this study suggests a new approach for the synthesis of various functional QDs probes for the detection of environmental pollution.
Declaration of competing interestNo conflict of interest exists.
AcknowledgementsThe authors would like to thank the grants from National Natural Science Foundation of Guangdong Province (Nos. 2017A030310666 and 2018A030307003), Guangdong Medical University Nanhai Marine Biomedical Resources R & D Public Service Platform Open Fund Project (Nos. 2HC18013 and 2HC18016), "Group-type" Special Support Project for Education Talents in Universities (No. 4SG19045G), Foundation of Young Innovative Talents in Guangdong Province Colleges (No. 2018KQNCX091), Undergraduate Science & Technology Innovation Foundation of Guangdong Province (Nos. 201810571046 and 201810571073), Medical Science and Technology Development Foundation of Guangdong Province (No. A2016355), The Opening Project of State Key Laboratory of Polymer Materials Engineering of Sichuan University (No. sklpme2018-4-23).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.029.
| [1] |
A.X. Xu, O.J. Scullen, S.S. Sheen, J.R. Johnson, C.H. Sommers, Food Microbiol. 84 (2019) 103264-103267. DOI:10.1016/j.fm.2019.103264 |
| [2] |
K.A. Krogfelt, P. Klemm, Microb. Pathog. 4 (1988) 231-238. DOI:10.1016/0882-4010(88)90073-3 |
| [3] |
G. Bonwick, C. Smith, J. Walliams, Food Sci. Technol. 17 (2003) 32-34. |
| [4] |
P. Belgrade, W. Benett, D. Hadley, et al., Science 284 (1999) 449-450. DOI:10.1126/science.284.5413.449 |
| [5] |
X.Y. Qu, Q.P. Wu, Z. Xiong, et al., Microbiol. China 7 (2019) 1-8. DOI:10.1186/s40168-018-0604-3 |
| [6] |
R.P. Liu, C. Wang, W.B. Xu, et al., Sensor Actuat. B -Chem. 6 (2013) 757-761. |
| [7] |
H. Mattoussai, J.M. Manro, E.R. Goldman, et al., J. Am. Chem. Soc. 122 (2000) 12142-12150. DOI:10.1021/ja002535y |
| [8] |
S.J. Sun, Q.W. Guan, B. Wei, et al., Chin. Chem. Lett. 30 (2019) 1051-1054. DOI:10.1016/j.cclet.2019.01.014 |
| [9] |
Z. Chen, C. Wu, Z.F. Zhang, et al., Chin. Chem. Lett. 29 (2018) 1601-1608. DOI:10.1016/j.cclet.2018.08.007 |
| [10] |
P. Zhao, Q. Xu, J. Tao, et al., WIREs Nanomed. Nanobiotechnol. 10 (2018) e1483. DOI:10.1002/wnan.1483 |
| [11] |
S.Y. Lu, G.J. Xiao, L.Z. Sui, et al., Angew. Chem. Int. Ed. 56 (2017) 6187-6191. DOI:10.1002/anie.201700757 |
| [12] |
S.Y. Lu, L.Z. Sui, J.J. Liu, et al., Adv. Mater. 29 (2017) 1603443-1603448. DOI:10.1002/adma.201603443 |
| [13] |
S.Y. Tao, S.Y. Lu, G.J. Geng, et al., Angew. Chem. Int. Ed. 57 (2018) 2393-2398. DOI:10.1002/anie.201712662 |
| [14] |
B.A. Rzigalinski, J.S. Strobl, Toxicol. Appl. Pharmacol. 238 (2009) 280-288. DOI:10.1016/j.taap.2009.04.010 |
| [15] |
N. Chen, Y. He, Y.Y. Su, et al., Biomaterials 33 (2012) 1238-1244. DOI:10.1016/j.biomaterials.2011.10.070 |
| [16] |
X.H. Xue, J. Pan, H.M. Xie, J.H. Wang, S. Zhang, Talanta 77 (2009) 1808-1813. DOI:10.1016/j.talanta.2008.10.025 |
| [17] |
D.P.L.A. Tenório, C.G. Andrade, P.E.C. Filho, et al., J. Photochem. Photobiol. B 142 (2015) 237-243. DOI:10.1016/j.jphotobiol.2014.11.010 |
| [18] |
C.I. Weng, H.T. Chang, C.H. Lin, et al., Biosens. Bioelectron. 68 (2015) 1-6. DOI:10.1016/j.bios.2014.12.028 |
| [19] |
H.Y. Chen, M. Zhang, B.W. Li, et al., Biomaterials 53 (2015) 532-544. DOI:10.1016/j.biomaterials.2015.02.105 |
| [20] |
Ü. Dogan, E. Kasap, D. Cetin, et al., Sens. Actuat. B -Chem. 233 (2016) 369-378. DOI:10.1016/j.snb.2016.04.081 |
| [21] |
B.Y. Wan, C.G. Hu, B. Feng, et al., Mater. Sci. Eng. B 171 (2010) 11-15. DOI:10.1016/j.mseb.2010.03.046 |
| [22] |
X.P. Wu, J. Gu, S.M. Zhou, et al., J. Alloys. Compd. 627 (2015) 166-173. DOI:10.1016/j.jallcom.2014.11.199 |
| [23] |
O.I. Olusola, M.L. Madugu, N.A. Abdul-Manaf, I.M. Dharmadasa, Curr. Appl. Phys. 16 (2016) 120-130. DOI:10.1016/j.cap.2015.11.008 |
| [24] |
S. Gao, G. Tang, D. Hua, et al., J. Mater. Chem. B. 7 (2019) 709-729. DOI:10.1039/C8TB02491J |
| [25] |
G. Tang, R. Xiong, D. Lv, et al., Adv. Sci. 6 (2019) 1802342-1802351. DOI:10.1002/advs.201802342 |
| [26] |
S. Li, S. Dong, W. Xu, et al., Adv. Sci. 5 (2018) 1700527. DOI:10.1002/advs.201700527 |
| [27] |
X. Zhao, Y. Han, T. Zhu, et al., J. Biomed. Nanotechnol. 15 (2019) 1213-1222. DOI:10.1166/jbn.2019.2773 |
| [28] |
X. Xing, F. Tang, J. Wu, et al., Anal. Chem. 86 (2014) 11269-11274. DOI:10.1021/ac502845b |
| [29] |
C.H. Xia, Q. Dai, W. Fang, H.S. Chen, J. Wuhan Univ. Technol. Sci. Ed. 29 (2007) 45-47. |
| [30] |
V. Biju, Y. Makita, A. Sonoda, et al., J. Phys. Chem. B 109 (2005) 13899-13905. DOI:10.1021/jp050424l |
| [31] |
M. Tomasulo, I. Yildiz, S.L. Kaanumalle, F.M. Raymo, Langmuir 22 (2006) 10284-10290. DOI:10.1021/la0618014 |
| [32] |
H.X. Zheng, B.W. Huang, J.M. Hu, Y. Gong, Acta Sci. Natural. Univ. Pek. 47 (2011) 777-782. |
| [33] |
M.M. Dzagli, V. Canpean, M. Iosin, M.A. Mohou, S. Astilean, J. Photochem. Photobiol. A:Chem. 215 (2010) 118-122. DOI:10.1016/j.jphotochem.2010.08.008 |
| [34] |
M. Konewaran, R. Narayanaswamy, Sensor Actuat. B -Chem. 139 (2009) 104-109. DOI:10.1016/j.snb.2008.09.028 |
| [35] |
E. Xu, Y. Xu, W. Liu, Z. Chen, J. Nanchang Univer. 25 (2001) 339-343. |
 2020, Vol. 31
2020, Vol. 31