b School of Material Science and Engineering, Beijing Institute of Technology, Beijing 100081, China;
c School of Education, College of Education, University of Rwanda, Rwamagana, P. O. Box 55, Rwanda
Currently, there is a high demand in research and development to design renewable energy and sustainable energy storage device due to the fast-growing population, decreasing fossil fuel sources, production of industrial waste for environment pollution. The development of devices with high energy, high power, and long cycle life has gained considerable attention to the research community. The supercapacitors have recognized many consider-ations for wide applications in energy storage device due to its outstanding performance mainly high power density delivery, rapid charge/discharge processes, better cyclic stability, and low-cost for maintenance [1-3]; long lifespan (> 100, 000 cycles) and safety; and broad working temperatures scales. Despite their wide benefits, there are still downsides in supercapacitors such as low energy density and high development cost compared with other energy storage devices like lithiumion batteries (LIBs) (180 Wh/kg for LIB while supercapacitor can deliver 10 Wh/kg) [4, 5]. A comparison of the LIBs and electric doublelayer capacitor (EDLC) over six important criteria of electrochemical energy storage devices was established as shown in Fig. S1 (Supporting information) [6].
Nowadays, the scientific research community and companies are both greatly incentivized to boost the energy density of supercapacitors by designing new active electrode materials, compatible novel electrolytes with a broad working voltage range, engineered device development. The different companies have contributed to the development and design of EDLCs with different shapes, operating voltage, energy-power density. Rapid progress has been undertaken on both materials and electrolytes to the enhanced energy density of EDLCs as the operating voltage of the commercial capacitor has been extended from 2.7 V to 3.0 V. The stable advance in supercapacitor research and development has revealed the opportunities for real-world applications.
The present review article does not only contribute to the environmental concerns of low-value plastic bag wastes (e.g., polyethylene, propylene, polystyrene, polyethylene terephthalate) but also propose a forward-looking idea for converting them into high-value supercapacitor-grade carbon materials with high yields via cost-effective technology and sustainable strategies. Moreover, we also give a short introduction of the developing path of the supercapacitor, including the evolution of different kinds of a supercapacitor. Furthermore, by using the new carbon as electrode materials in high-voltage symmetric and hybrids supercapacitors in both aqueous and non-aqueous electrolytes, energy storage devices with high energy density and superior cycle stability are implemented.
1.1. EDLC and other supercapacitors 1.1.1. Mechanism for EDLCsThe supercapacitor is mainly made of two electrodes, electrolytes (an aqueous or an organic system), a separator that allows the migration of electrolyte ions while electrically separating the two electrodes, fuses and packaging materials, and so on. The EDLCs are built much similar to a battery in which there are two electrodes soaked in an electrolyte, with an ion-permeable separator located between the electrodes.
In 1853, Hermann von Helmholtz explained the concept of the doublelayer capacitance (C) for the first time stipulating that charge separations happen on polarization at the interface of electrode-electrolyte and form two layers, which are separated by an atomic distance as presented in Fig. S2a (Supporting information). The model is similar to that of two-plate conventional capacitors [7-9]. The Helmholtz doublelayer (DL) can be considered as an electrical capacitor of capacitance CH from the following equation:
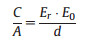
|
where Er is electrolyte dielectric constant; E0 is the dielectric constant of the vacuum; d is the effective thickness of the double layer (charge separation distance); A represents the electrode surface area. Regarding the high specific surface area of porous carbon electrodes (up to 3000 m2/g) and Debye length in the range of < 1 nm, the resulting capacitance of DL's will be much larger than for flat plate capacitors.
In 1910, this simple model which considers the diffuse layer was modified by Gouy and Chapman, as shown in Fig. S2b (Supporting information), formed by electrolyte ions as a result of thermal motion wherein the potential decreases exponentially from the electrode surface to the fluid bulk [10]. However, the Gouy-chapman model leads to an overestimation of the EDLC capacitance. In 1924, Stern combined the Helmholtz model with the Gouy-Chapman model to explicitly recognize two regions of ions distribution, an inner region called the compact layer or Stern layer and a diffuse layer as shown in Fig. S2c (Supporting information) [11, 32]. The inner Helmholtz plane (IHP) and outer Helmholtz plane (OHP) are used to distinguishing the two types of adsorbed ions.
When an electric potential difference is applied between the electrodes, the negative charge carriers, electrons, in the negatively polarized electrode are balanced by an equal number of positive cations at the electrode/electrolyte interface, while the holes stored at the positively polarized electrode are electrically balanced by anions. Hence, a supercapacitor consisting of two electrodes is equivalent to two capacitors in series and the resulting capacitance (C) can then be expressed according to 1/C = 1/C++1/C-, where C+, C- and C are the capacitance (F = C/V) of the positive electrode, the negative electrode, and of the resulting device, respectively. The doublelayer capacitance is between 5 μF/cm2 and 20 μF/cm2 relying on the type of electrolyte [11]. Generally, EDLCs can be operated at a very high charge and discharge rate with a lifetime of over a million cycles. However, the energy stored in the EDLCs is lower than that in batteries.
1.1.2. Mechanism for lithiumion capacitors (LICs)LICs are electrochemical energy storage devices that combine the advantages of the high power density of a supercapacitor and the high energy density of a Liion battery. LICs are usually composed of a battery-type electrode with the insertion/extraction of Li ions and a capacitor-type electrode with pseudo-capacitance or adsorption/desorption of ions [12-14]. Since the battery-type electrode can not only act as an anode but also serve as a cathode, the LICs can be divided into two types: (1) The battery-type electrode acts as the anode, and the capacitor-type electrode serves as the cathode like a Li4Ti5O12//AC system. Typically, during the charging process, anions are absorbed on the porous surface or defects of the cathode, while Li+ ions are inserted into the active material of the anode. During discharging, the adsorbed anions are released from the cathode, and the Li ions are extracted from the anode [15]; (2) The capacitor-type electrode acts as the anode, and the battery-type electrode serves as the cathode like an AC// LiFePO4 system. Typically, during the charging process, Li+ de-intercalates from the cathode material and enters the electrolyte. At the same time, Li+ in the electrolyte migrates and adsorbs on the anode. The discharge process will be the reverse case as presented, and another example was also offered for illustration of the electrostatic and electrochemical charge and discharge of the double layer and intercalation function in the fabricated hybrid supercapacitor consisting of rGO/LTO and graphite (Fig. S3 in Supporting information) [16].
1.1.3. Classification of supercapacitorsThe supercapacitors can be classified into several classes owing to the energy storage mechanism and the active materials utilized. The first one is EDLCs, and the second one is pseudocapacitors, but there are other classes such as hybrid capacitors and battery-type capacitors (Fig. 1A).

|
Download:
|
| Fig. 1. (A) Types of supercapacitors (SCs) and their operating potential window, Courtesy of Prof. Zhiqiang Shi, (B) Voltage profile for an EDLC and Lithiumion capacitor (LIC). Reproduced with permission [17]. Copyright 2018, The Royal Society of Chemistry. | |
The EDLCs can store the charge electrostatically employing reversible adsorption of ions of the electrolyte on the electroactive material which is electrochemically stable and possesses great accessible specific surface area. The high surface area of the EDLCs electrodes coupled with a small thickness of the double layer results in a specific and volumetric capacitance two orders of magnitude larger than that of the electrolytic capacitors. The electrolytic capacitors provide excellent power density features at the expense of lower energy density. Also, EDLCs can deliver ultrahigh power density and an excellent lifespan due to the non-degrative processes between the electrode and the electrolyte.
Typical voltage profiles for an EDLC cell and an LIC cell; schematics of the EDLC with activated carbon electrode as both positive and negative electrodes; conventional hybrid capacitor with a battery negative electrode and activated carbon (AC) positive electrode and nanohybrid capacitor with a pseudocapa-citive or an ultrafast nano-structured battery negative electrode combined with an AC positive electrode were developed as presented in Fig. 1B [17, 18].
The pseudocapacitors, where the fast and reversible redox reactions between the electrolyte and electroactive species on the electrode surface. Pseudocapacitors are, thus, suitable in applications where high capacitance is needed. The energy densities of pseudocapacitors are higher than EDLCs, but the phase changes within the electrode due to the faradaic reaction limit their lifetime and power density. The operating potential window (OPW) of pseudocapacitor is low (< 1.2 V), which is still affecting their energy density mostly needed for its widespread applications. The pseudocapacitors use the materials possessing redoxactive transitions metals with different oxidations states like metal oxides, RuO2, Fe3O4, NiO and MnO2, electronically conducting polymers such as polyanilines (PANIs), polypyrrole (Ppys), poly-thiophenes [19, 20] and heteroatoms-doped carbon materials. To address the phase changes issues, carbonaceous materials like carbon nanotubes and graphene with high electrical conductivity, ductility have been used to synthesize composites with pseudo-capacitive materials [21-23]. It is worth noting that due to the low cost, quick rate, longer lifespan, and low-self discharge, EDLCs-type supercapacitors currently dominate the market while the recent market fraction for the pseudocapacitors is so small.
The third category is hybrid supercapacitors with asymmetrical electrode configuration, where one electrode consists of electrostatic absorbed carbon materials while the other consists of faradaic electroactive materials (pseudocapacitance). The hybrid supercapacitors are classified into LICs, which can work in voltage ranged from 2.2 V to 3.8 V, and the nanohybrid capacitors (NHCs) can work and stable in electrochemical voltage window between 1.5 V and 2.8 V. These asymmetric configurations characterize an increased working voltage with a high energy density and a high power density. Therefore, it is necessary to design and develop the hybrid system to address the energy density limitations of the present generation of carbon-based supercapacitors because they use both the system of a battery-type (redox) and a capacitor-type (doublelayer) electrode, which will allow achieving a wide operating voltage and capacitance. The two various technique to hybrid supercapacitor has appeared (1) pseudocapacitive metal oxides with a capacitive carbon electrode, and (2) lithium-insertion electrodes with a capacitive carbon electrode [2]. Furthermore, asymmetric configurations demonstrate enhanced specific energy at the expense of rate performance because the battery behavior principally results from faradic redox reactions with the crystalline framework of the active materials [24].
LICs are the most interesting hybrid device due to their high OPW increased from 3.8 V to 4.3 V with high energy density up to 25-40 Wh/kg. Also, the LIC is a hybrid of a LIB negative electrode and EDLC positive electrode. Li+ insertion-extraction occurs in the graphite electrode with shallower state of charge (SOC, less than 50%) compared with the LIB system, while adsorption-desorption of anions, most typically BF4- or PF6-, occurs on the AC electrode, as in the EDLC. The overall process is a cation-and anion-consuming reaction [6]. The LIC has a specific energy and energy density of around 30 Wh/kg and 20 Wh/L, respectively. These values are more than three times higher than the values of a conventional EDLCs. Later, Kim et al. proposed a concept for LIC, Li2MoO3 is integrated into the cathode as a lithium-doping agent, which Li+ can be reversible extracted, and then inserted into the anode [25, 26]. In dual carbon lithiumion capacitor, the socalled dual carbon type of LICs use a high surface area AC material as the positive electrode and a lithiumion intercalating carbon material such as graphite or coke as a negative electrode. They can store about five times more energy than conventional EDLCs while keeping good power and long lifespan characteristics [26]. During charge/discharge, lithi-umion insertion/extraction occurs with the bulk of the negative electrode, whereas anion adsorption/desorption occurs on the surface of the AC positive electrode. As the latter process on the positive electrode is non-faradic and relatively rapid in comparison with the lithiumion exchange process at the negative electrode, the power capability of the LIC will generally be determined or limited by the rate capability of the negative electrode.
NHC is the second class of hybrid supercapacitors. In 2001, G.G. Amatucci and co-workers first suggested the nanohybrid capacitor, where Li4Ti5O12 (LTO) was used as a negative electrode combined with AC as a positive electrode in acetonitrile electrolyte [27] to reach 3 V high voltage, energy density of 20 Wh/kg and cyclic stability for 4000 potential cycles. In 2009, Naoi and co-workers developed a hybrid supercapacitor system that attained a high energy-power density and high stability, whereas keeping safe. The socalled "nanohybrid capacitor employs an ultrafast anode electrode based on nanoscale Li4Ti5O12 crystals, which were highly-dispersed and entangled within a nano-carbon framework. The authors revealed that LTO is stable and safe redox materials able to raise the energy density of hybrid supercapacitors without sacrificing the interfacial characteristics. LTO can work at a voltage of 1.55 V (vs. Li/Li+), which is outside of the voltage range where the electrolytes decompose. Others benefits of LTO in nanohybrid capacitors are (i) high coulombic efficiency (> 95% at 1 C) very close to a theoretical capacity of 175 mAh/g, (ii) zero-strain insertion that provides little volume change during charge-discharge, (iii) little electrolyte decomposition (little SEI formation and little gas evolution) and (iv) cheap raw material [28]. For example, Katsuhiko Noi et al. have used ultrafast materials to assemble the nanohybrid capacitor, which consisted of a faradaic Li-intercalating LTO electrode and a non-faradaic AC electrode using an anion (typically BF4-, anion from 1 mol/L LiBF4 dissolved in ethylene carbonate (EC) and dimethyl carbonate (DMC)) adsorption-desorption process [25].
The assembled nanohybrid cell has exhibited remarkable energy, power, and ability performance as an electrochemical capacitor. Furthermore, the nanohybrid technology produced more than triple the energy density of a conventional electrochemical capacitor.
There is a large selection of electrolytes for NHC because of its narrower voltage window (2.7-3.0 V) compared with the LIC system (4.0-4.3 V). Acetonitrile (AN), ionic liquids (ILs) and linear carbonates, such as DMC or diethyl carbonate (DEC), can be utilized in NHCs. The selection of electrolytes is very important to obtain an even better power performance. There is another class of excellent supercapacitors configuration is micro-supercapacitors [29], an important prerequisite for the fast development of miniaturized, wearable, and portable electronics have been developed micro-power sources on chips. A comparative study of LIC and NHC on working mechanism was performed (Fig. S4 in Supporting information) [6].
The battery-type capacitor, where rechargeable battery electrodes influenced the development of electrodes for new hybrid-type supercapacitor electrodes as for LICs and battery-type capacitors. Together with a carbon EDLC electrode in an asymmetric construction offers higher specific energy than typical super-capacitors with higher specific power, longer cycle life, and faster charging and recharging times than batteries. The benefit of the battery-type capacitor is their higher voltage ranged from 1.5 V to 4.2 V and corresponding to their higher specific energy up to 10-20 Wh/kg. Research group of A. Yoshino, T. Tsubata et al. [30] have used a composite electrode prepared by heat treatment on an AC with pitch as the negative electrode in their device.
The Li+ intercalation/deintercalation of the negative was shown to occur in a potential range similar to that of graphite but with enhanced kinetics. The the-assembled device worked in the voltage window of 2.0-4.0 V, and its power 2.2 kW/L and energy density of 20 Wh/L has been claimed to be two to three times higher than EDLCs.
1.2. Electrolytes for EDLCsThe electrolyte plays an important role in the energy storage device, and it can contribute to the enhanced performance of supercapacitors. The OPW is normally hampered by the electrochemical stability of the electrolyte. The cell voltage is also an important determinant of the specific energy and power of ECs as their final operating voltage relies on electrolyte stability. There are three types of electrolytes that can be used in EDLCs: (1) aqueous, (2) salts dissolved in organic solvents, and (3) ionic liquids (Fig. 1A).
The EDLCs-based on the aqueous electrolytes, the aqueous electrolytes have a high ionic conductivity up to 1 S/cm, low cost, and wide acceptance (i.e., H2SO4 and KOH). The specific capaci-tance of carbon materials in the aqueous electrolytes is generally greater than the one achieved for the same electrode in non-aqueous electrolytes. The drawbacks of the aqueous electrolytes are corrosive at higher temperatures; their voltage window cannot exceed 1.0 V because of water decomposition, contributing to the self-discharge, and are suitable for the demand for high power or low-cost device only. While the low working potential of aqueous systems leads to a significant limitation if the high energy is required.
EDLCs-based on organic electrolytes, the organic electrolytes are more broadly applied for symmetric systems because they can work at higher operating voltage window up to 2.7 V correlating to the energy density in supercapacitors. The same advantage can be transferred to the battery-type capacitor by the same adoption of organic electrolytes. Organic-based EDLCs offer cycle life over 500, 000 and are used in the majority of commercials EDLCs. Moreover, EDLCs with organic electrolytes are much less flamma-ble than lithiumion batteries. Albeit organic electrolytes can offer large working voltages beneficial for supercapacitors, they present the shortcomings like maintenance (volatile, tedious purification processes), higher environmental impact, higher cost (both materials and manufacture), safety issues (explosion risks due to the poor thermal stability, etc.), and low ionic conductivity (diminished power capability). The high voltage organic devices are highly appealing for high-energy applications, and the electrolytes mixtures of solvents, containing dissolved quaternary alkylammonium salts, are routinely used in many commercial supercapacitors. The most common organic electrolytes for EDLCs is alkyl ammonium salt dissolved in an aprotic solvent. For example, 1 mol/L TEABF4 has an ionic conductivity of 60 mS/cm when dissolved in acetonitrile and around 11 mS/cm when the less volatile propylene carbonate (PC) solvent is used. Thus, the valuable step has been achieved by shifting from aqueous to non-aqueous electrolytes, with a dramatic gain in energy density further enhanced the potential operating window. For example, Zhiqiang Shi and co-workers from Tianjin Polytechnic University have successfully synthesized a novel electrolyte salt spiro-(1, 1')-bipyrrolidinium bis(fluorosulfonyl) imide (SBP-FSI) via the one-step method in an aqueous solvent by a simple and green procedure.
The as-prepared SBP-FSI/PC achieved a working voltage of 3.2 V to effectively compensate for the loss of ion mobility in low temperature (-40 ℃), highly enhancing the energy density and power density of supercapacitors. Moreover, the as-prepared the innovative electrolyte system allowed EDLCs to display high stability at 3.2 V, delivering the highest energy density of 42.67 Wh/kg and power density of 5951 W/kg at -40 ℃, achieving a substantial improvement in low-temperature cycling performance with 88.92% capacity retention after 10, 000 cycles (Figs. S6a-c in Supporting information) [31].
Another example was given, a comparison of the EDLCs operating voltage achievable with organic electrolyte and ionic liquids based electrolytes. AN (acetonitrile), PC, ADN (adiponitrile), Alkylat. Cyc. Carb. (alkylated cyclic carbonate), EC (ethylene carbonate), DMC, LiPF6 (lithium hexafluorophosphate), ionic liquids, as shown in Fig. S5 (Supporting information) [32].
EDLCs-based on ionic liquids electrolytes, ILs electrolytes are used for increasing operating voltage as high as 4 V and for developing high energy EDLCS capable operating beyond room temperature safe in operating systems and have good conductivity over room temperature, thereby giving a chance to develop safe, "green" high voltage EDLCs free of toxic and/or flammable organic solvents and able to work even at high temperature ranges between 60 ℃ and 80 ℃. The ionic liquids are a class of organics that are liquid at relatively low temperatures (< 100 ℃) and can be used as solvent-free electrolytes, thereby avoiding the flammability and volatility concerns often associated with organic solvent-based electrolytes. The viscosity values of ILs are significantly higher than those aqueous solutions. The ILs at room temperature feature conductivities of 0.1-14 mS/cm, which can even be two orders of magnitude lower than those of aqueous electrolytes (400-700 mS/cm). However, the ionic conductivity of RTILs, especially at room temperature, is generally lower than that of organic electrolytes, reducing the power performance of RTIL-based supercapacitors.
The ionic liquids electrolytes are used in supercapacitors at a laboratory scale because of their high price, high molar mass, and low transport properties such as high viscosity and low ionic conductivity, which reduce the performance of supercapacitors. Therefore, to reduce the shortcomings of pure ionic liquid, it is better for future research to combine organic solvents (propylene carbonate or acetonitrile for keeping a large voltage stability window) and ionic liquids are considered as an ideal approach. The main benefit of propylene carbonate over acetonitrile is its less toxicity, which will help supercapacitor to fulfill the industrial requirement for winning the future market. The use of the ionic liquid electrolytes is a good choice during the development of EDLCs due to their higher working potential window than aqueous electrolytes and organic electrolytes [32]. Recently, Yu and Chen have reviewed progress on the capacitive electrochemical energy storage (EES) devices, including supercapacitor, battery-type capacitor, and micro-supercapacitors from the perspective of ionic liquid electrolytes. They found that a battery-type capacitor on ILs electrolytes are gradually becoming competitive compared with other EES devices when high-performance energy storage devices are strongly required nowadays. All progress and efforts made from the research and industrial communities so far are promising a bright future for supercapacitors and battery-type capacitors [33].
Most research reports focus on energy density boosting, but the champion is not one of the highest energy density. The other crucial factors should be considered, like cost, environment threatens, and other types of applications. Among them, there are the binders that are also used to glue the individual parts and keep them together to fabricate the electrode as a result.
The most used binders in the research community and industry are polyvinylidene fluoride (PVDF, 1.76 g/cm3), polytetrafluoro-ethylene (PTFE, 2.2 g/cm3) and sodium carboxymethyl cellulose (1.6 g/cm3). The binders containing fluorine in their chain are expensive, possess high density, and release the fluorine, which creates environment incompatibility, very hard to tackle after the device's lifetime. The promising candidate of binder should thus offer good adhesion at a little binder amount; they should be inexpensive, and mechanically stable electrode with high mass loadings. For future research and to win the global market of the supercapacitor, a new research area should focus on the design and development of fluorine-free binders, which are a low-cost and safe environment.
1.3. The requirement for high-performance carbon materials for EDLCsThe carbon is the one most plentiful resource naturally and structurally different materials, in which activated carbons are the mostly applied for EDLC use. The studies have pointed out that the general properties of high-performance carbons materials for EDLC applications: (1) High surface area like greater than 1500 m2/g; (2) High bulk density such as the specific pore volume smaller than 1.0 mL/g; (3) High specific mesopore fraction. The pore volume distribution of 0.4 mL/g or greater for the pores in the range between 12 nm and 40 nm in diameter and of 0.5 mL/g or lower for the pore greater than 40 nm; (4) High conductivity like greater than 1.0 S/cm; (5) High purity (99.9% carbon, ash content lower than 0.1%); (6) High cost-performance. The International Union of Pure and Applied Chemistry (IUPAC) describes the pores based on their size as macropores: greater than 50 nm; mesopores arranged from 2 nm to 50 nm and micropores: lower than 2 nm [34]. In 2016, Cao and co-workers from the Research Institute of Chemical Defense (Beijing, China) reviewed the mechanism of the energy storage for EDLCs. They demonstrated that the more effective technique to attain high power density and high energy density for porous carbon-based supercapacitors is to develop the porous carbon with optimal micropores size and appropriate proportional of mesopores (Fig. S7 in Supporting information) [35, 36].
The carbon materials with mesopores and macropores boost energy density and power density of the EDLC. The crucial factor for achieving high capacitance by charging the double layer capacitor is employing high specific surface area blocking and electronically conducting electrodes. Graphitic carbon fulfills all these conditions, including high conductivity, electrochemical stability, and open porosity [37]. The electronic and ionic conductivity are also among the essential parameters for super-capacitor evaluation [38]. The activated carbons are the most broadly employed materials today in industry and scientific research community due to their high specific surface area and affordable price.
The total price of carbon materials consists of a 50% cost of supercapacitor was dominated by coconut shell-derived activated carbon. And oxidizing agents such as steam and carbon dioxide at high-temperature are always introduced in the manufacturing processes to activate and char the biomass precursors. The price of the coconut shell-derived activated carbon has considerably dropped from $100 to $15 per kg, which causes the barrier to the introduction of other carbon materials in the field [39]. In supercapacitor applications, energy density is proportional to the product of specific capacitance and square of the voltage. Therefore, there are two strategies for new type carbon to replace the coconut shell-derived activated carbon in the field. Either developing new type carbon with high working potential of 3.5-4 V in contrast with the coconut shell-derived activated carbon performing at 2.7 V, and thus, double the energy density of the supercapacitor; or developing low-cost and facile synthetic processing for the corrosiveness of the carbon precursors, and low-cost for fabrication of supercapacitor-grade activated carbon. For the scientific researchers, plastic bag wastes are considered as alternatives carbon precursors to coconut shells for preparing carbon electrodes for supercapacitors due to their high carbon content, low-cost, and abundance in the environment.
1.4. Plastic waste and other industrials wastesNowadays, the ever-growing of human life makes the plastic materials to be more and more used in business and by consumers due to their convenience, low cost, high strength, durability, resistance to corrosion, and lightweight but after use they become a burden to the environment and wildlife health due to the inappropriate technology to handle them and take several years to be degraded, or demand expensive, complex strategy and thus, some countries establish laws to restrict littering plastics waste. Murat Barsbayc et al. state that plastics are indispensable in our everyday lives and will continue to be so; we have to search better ways to responsively and sustainably design, recycle and recover them [40]. Therefore, more plastic waste and other industrial wastes such as compact discs, printed circuits boards, tires, coal tar pitch, solid leather, are produced. Also, more than 50 million tones of polyethylene terephthalate (PET) hard to biodegradation, accumulated in the ecosystem around the earth, is produced every year [41]. The plastics waste ended up in the landfills, in the oceans or elsewhere in the environment will give off the toxic substances in the environment and may pollute the water, harm human life, as well as wildlife health while incinerated ones emit carbon dioxide and toxic gases due to the lack of appropriate technologies to dispose of them. Therefore, there is an urgent call from human communities and environment to waste-management companies, scientific researchers, and entre-preneurs to find sustainable technology to dispose of plastic wastes properly for better human health future and economic development. Various carbonization methods such as pyrolysis and activation techniques are required for the production of carbon materials from different carbon precursors (Fig. 2).
|
Download:
|
| Fig. 2. Diagram for converting plastic waste and other industrial waste into electrode materials for supercapacitor. | |
The physical and chemical techniques both can be used, or joint of the two methods have been developed for transforming industrial wastes into high value-added carbon materials for supercapacitors. Therefore, they can be explored and then generate high value-added products. Therefore, they are "raw materials" instead of "waste". Active carbon materials with different surface properties, morphologies, and various porosities can be produced by paying attention to temperature, time, and chemical agents used during the synthesis.
2. Preparation methods for carbon electrode materials 2.1. Activation methodsActivation is considered as a process of turning raw materials from industries into active materials. The pyrolysis method is commonly used to convert industrial waste into activated carbon. The pyrolysis occurs in inert or less oxygen atmosphere at high temperatures for turning industrial waste to carbonaceous materials [42].
2.1.1. Physical activation methodPhysical activation usually happened directly after the pyrolysis step at elevated temperatures up to 1200 ℃ in a steam or carbon dioxide (CO2) atmosphere [43, 44] or their combinations. The physical activation is generally performed in a gas such as air, steam, or CO2. In the physical activation process, a carbon source is first heated in an inert atmosphere between 400 ℃ and 900 ℃ to remove bulky volatile materials-subsequently, partial gasification employing an oxidizing gas at temperature ranged from 350 ℃ to 1000 ℃. Firstly, the active oxygen in the activating agent burns away the tarry pyrolysis off-products embedded in the pores, conducting to the opening of some clogged pores. Secondly, the microporous structure is developed as the oxidizing agent burns away more reactive areas in the carbon framework resulting in CO and CO2. The extent of annealing is relying on the use of gas and activation temperature. The CO2, air, and steam are employed as activating agents [45-47]. The steam is an abundant activating agent employed for turning industrial raw material owing to its low value and no subsequent activation process to eliminate useless products. The steam is usually mixed with pyrolysis as one-pot. It can produce rich surface oxygen-functionalities, which enhance the wettability and capacitance of the produced carbon materials.
2.1.2. Chemical activation methodThe chemical activation is performed in the presence of chemical activating agents at a temperature ranged from 450 ℃ to 900 ℃ but combining the carbon source with alkalis, carbonates, chlorides, acids, or same agents (KOH, NaOH, K2CO3, ZnCl2, FeCl3, H3PO4 and H2SO4) [44]. The chemical activation is greatly carried out at the laboratory level and provides benefits in pore size distribution control. Also, the chemical activation is a one-pot process, where an activating agent such as an acid, strong base, or salt is embedded into carbon precursor before pyrolysis at a temperature ranged from 450 ℃ to 900 ℃. The chemical activation has downsides such as much water required for washing away the impurities produced during activation and the management of polluted water [48]. The alkalis like KOH is mostly employed for activating the industrial raw materials-derived active materials for supercapacitors applications [49]. The chemical activation of various carbon sources with KOH activating agent is very promising method due to its lower activation temperature, short activation time, higher yields, and well-defined micropore size distribution, and as well as the ultrahigh specific surface area of the resulting porous carbons that are very important in charge storage. In the KOH activation method, different reactions mechanisms have been proposed for clarifying the preparation of activated carbon materials for supercapacitors. Those mechanisms are as follows:
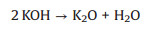
|
(1) |

|
(2) |

|
(3) |

|
(4) |
Linares-Solano and co-authors reported that the evolution of CO2, CO and H2 was observed during KOH activation via the temperature-programmed reaction (TPR) experiments [50, 51]. The prominent global reaction stoichiometrically happening between carbon and KOH is suggested as presented in the following equation:
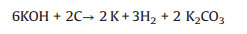
|
(5) |

|
(6) |
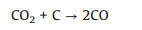
|
(7) |
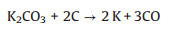
|
(8) |
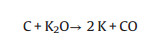
|
(9) |
During KOH activation, K2CO3 produces at about 400 ℃ [52]. At about 600 ℃, KOH is totally used up. The as-produced K2CO3 in reactions (4) and (5), were significantly transformed into CO2 and K2O at temperatures higher than 700 ℃ (reaction (6)), totally vanishes at around 800 ℃. Furthermore, the resulting CO2 can be further reduced by carbon to form CO at high temperatures (reaction (7)). The K2O and K2CO3 also can be reduced by carbon to produce metallic K at temperatures over 700 ℃ (reactions (8) and (9)). Based on the results presented in literature [53, 54], there are three main activation mechanisms for KOH activation of carbon which are: (i) Etching the carbon framework by the redox reactions between various potassium compounds as chemical activating reagents with carbon as shown in reactions (5), (8) and (9), called chemical activation, is responsible for generating the pore network; (ii) The formation of H2O (reaction (1)) and CO2 (reactions (3) and (6)) in the activation system positively contributes to the further development of the porosity through the gasification of carbon, namely physical activation (reactions (2) and (7)) [55]; (iii) The as-formed metallic K (reactions (5), (8) and (9)), efficiently intercalating into the carbon lattices of the carbon matrix during the activation, results in the expansion of the carbon lattices [56]. After the removal of the intercalated metallic K and other K compounds by washing, the expanded carbon lattices cannot return to their previous nonporous structure and thus the high microporosity that is necessary for large specific surface area, and pore volume (PV)/micropore volume (MV) is created. For a given carbon precursor, experimental variables of KOH activation include the mass ratio of KOH/ carbon precursor, heating rate (3-10 ℃/min), activation temper-ature and time (1-4 h). It is worth noting that excess activation can cause a larger pore volume, which further makes the density and conductivity of the activated carbon decrease. Therefore, a lower volumetric energy density and loss of power capability are produced. So finely tailoring the porous microstructure and surface chemistry of porous carbons by KOH activation is crucial for obtaining high-performance supercapacitors while balancing the gravimetric and volumetric capacitances. The substantial efforts have been used for synthesizing and tailoring carbon microstructures materials for energy storage. For instance, Wang and Kaskel have reported the KOH Activation of carbon-based materials for energy storage [57]. From their deep analysis, they have concluded that: (i) The large micropore of the activated carbon is formed when the mass ratio of KOH-carbon and activation temperature raise; (ii) The integration of micropores, and small mesopores into the different carbon nanostructured framework by KOH activation highly increases the interconnected pore networks and porosity, while keeping the original textural properties; (iii) The carbon structure, pore size, and surface functionalities are among key factors which can affect the supercapacitor performance. For instance, the normalized capac-itance decreases with the increase of micropore size. Moreover, the introduction of heteroatoms by using nitrogen/oxygen-riched precursors can enhance the specific capacitance via the pseudo-capacitance effect. It is worth noting that long activation time or elevated temperature conducts to larger average pore size. The synergism of physical and chemical processes is also feasible. There are great advantages of using KOH such development of a high surface area and high porosity because of synergism and comprehensive actions including chemical activation, physical activation and carbon lattice expansion by the metallic K intercalation. The higher specific surface area plays a crucial role in EDLC performance, as the capacitance increases linearly with it approved by Helmholtz [8]. For instance, He and Qiu's group have prepared mesoporous carbons (MCs) for supercapacitors by using coal tar pitch throughout a microwave-assisted one-step process coupling the KOH activation and MgO template at only 30 min of heating [58]. The-obtained MC displayed a specific surface area of 1394 m2/g and a pore volume of 0.83 cm3/g and showed a high specific capacitance of 224 F/g in 6 mol/L KOH electrolyte after 1000 cycles. In comparison, the two-step activation approach is frequently applied for synthesizing plastic waste derived carbon electrode materials for supercapacitor applications. He et al. have used low-density polyethylene (LDPE) to synthesize a hierarchical porous carbon (HPC) through autogenic pressure carbonization at 600 ℃ at 10 ℃/min in a tube furnace followed by KOH activation at 700 ℃ at a rate of 5 ℃/min under nitrogen atmosphere [59]. The as-prepared HPC exhibited a large specific surface area of 3059 m2/g, a pore volume of 1.73 cm3/g, abundant surface functional groups, and used as an electrode material for supercapacitors.
ZnCl2 is another commonly used chemical activating agent for turning industrial wastes into activated porous carbon materials for supercapacitors. ZnCl2 plays the role of a dehydrating agent during the activation process, and it also has a deoxygenation effect at high temperatures by removing oxygen in the form of water as well as by carbothermal reduction. Some activated carbons with high performance derived from industrial waste have been reported throughout a simple one-step process and ZnCl2-activating agent. In a research performed by Chang and Yang's research group, the hierarchical activated porous carbon (APC) was synthesized through a chemical activation route using ZnCl2 and recycled waste filter paper (FP) as the carbon precursor [60]. The optimal sample, APC-7-4, was activated at 700 ℃ with a ZnCl2/carbon precursor mass ratio of 4:1 and presented a large surface area of 2170 m2/g, a suitable pore size of 1.5 cm3/g. Significantly, when the as-prepared APC-7-4 material used as the electrode material for supercapacitor, APC-7-4 demonstrated an outstanding charge storage capacity with a satisfactory specific capacitance of 302.3 F/g in 6 mol/L KOH at 1 A/g.
Phosphoric acid (H3PO4) can also serve as a chemical activating agent. Compared with KOH and ZnCl2, H3PO4-activated carbon usually possesses a relatively low specific surface area and small pore volume. For example, the waste-tires were used as carbon precursors for preparing activated carbon and H3PO4 as an activating reagent, after activation with H3PO4/char ratio of 5 at 900 ℃ for 3.5 h under nitrogen atmosphere, the obtained waste tire activated carbon (WTAC) displayed a high specific surface area of 563 m2/g and pore volume of 0.2 cm3/g, when used as electrode material for supercapacitor showed a specific capacitance of 106.4 F/g [42].
Over and above KOH, ZnCl2, H3PO4, and others like ammonia (NH3) are also applicable for preparing activated carbon materials derived from plastic bag waste and other industrial waste for supercapacitor application. In 2019, Yang's research group has designed an ingenious method for converting low-cost polyeth-ylene plastic waste into a mesoporous carbon material with a remarkable high surface area of 1219 m2/g and pore volume of 2.0 cm3/g via carbonization at 900 ℃ in N2 for 1 h and further activated in ammonia for 2 h with different temperature, where the optimized one was 900 ℃ [61]. The-obtained mesoporous carbon materials displayed a specific capacitance of 244 F/g at 0.2 A/g, and still 125 F/g even at 50 A/g in 6 mol/L KOH electrolyte, again confirming the good rate capability. The prepared meso-porous carbon was used in assembling symmetrical super-capacitor with EMIMBF4 electrolyte delivered a maximum energy density of 43 Wh/kg. Another method to synthesize the hierarchical porous carbon with mesopores and a high surface area of 1381 m2/g was also developed by Yang et al. [62] by using ionic liquid confined in the metal-organic framework by an in-situ ionothermal synthesis throughout carbonization and further activated in ammonia. Their method is also applicable for preparing the hierarchical porous carbon with mesopores for supercapacitor applications.
2.2. Template-based methodThe different templates are used for preparing an activated carbon material such as hard templates, soft templates, or the mixture of both templates, and zeolites as a porous sacrificial structure-directing agent are usable for converting industrial raw materials into carbon-based materials for supercapacitors appli-cations.
2.2.1. Hard template-based methodThe hard template-based methods used hard particles, such as SiO2 and polymer colloids, as a sacrificial template to build the macroporous structure. Typically, the empty spaces between colloidal particles in the templates are infiltrated by a fluid-like carbon precursor that penetrates the templates and can be turned into a solid. Next, under pyrolysis treatment, solid fillers are turned into carbons under an inert atmosphere, and the templates are eliminated by pyrolysis or chemical etching. The solid carbon framework is formed after removing templating particles that surround the air holes (macropores) left in the original locations of the particles [63]. If the KOH activation process is subsequently performed, hence hierarchically porous carbon structure is produced. Besides, when heteroatoms-rich compound (Boron, N-containing ionic liquid, melamine, P-, S-based compounds) is combined with a precursor; therefore, the heteroatoms doped-hierarchical porous carbon structure can be synthesized.
2.2.2. Soft template-based methodThe soft template-based methods present some quite appealing benefits like its ability to prepare carbon materials with different morphologies and less harsh experimental conditions. We can also use the heteroatom rich compound for synthesizing the doped hierarchical porous carbon material for supercapacitor electrodes. For preparing the hierarchical porous carbon materials with the coexistence of macropores and mesopores, which are beneficial to good electrochemical performances of supercapacitors, it is better to mix soft-hard-templating method. In a typical run, hard particles and the micelles of triblock copolymers (F127) can be used as the macroporous and mesoporous templates, respectively. Owing to the little space of hard templates, the soft templates occupy the interstices between hard colloids with relatively large size. The benefits of macropores-based hierarchically porous carbon electrode contain not only facilitating electrolyte access and ions transport but also enhancing the loading and dispersion of active materials, thus fostering capacitance generated by the supercapacitor.
2.2.3. Template-free methodMany efforts have been used for synthesizing porous activated carbon material derived from carbon precursors coupled with various templates for supercapacitor applications, but also the hierarchical porous activated carbon materials can be prepared without introducing any templates. The template-free methods hold the following advantages, such as short-time, simple and affordable prices compared with template-based methods.
For example, Li and Zhao et al. have first designed an effective and simple self-assembly method without adding templates to prepare layer-stacked hierarchical porous activated carbons (LHPCs) from coal tar pitch [64]. The as-prepared LHPCs showed a super large specific surface area of 3114 m2/g, a large pore volume of 2.0 cm3/g, and exhibited excellent electrochemical perform-ances with a high specific capacitance of 356.8 F/g at 0.5 A/g in 6 mol/L KOH electrolyte.
Also, symmetrical all-solid-state supercapacitor was assembled using LHPCs achieved excellent flexibility and outstanding cycling stability up to 100, 000 cycles, as well as a high energy density of 10.25 Wh/kg at a power density of 496 W/kg. There is a new way to the controllable synthesis of activated carbons from coal tar pitch and would also be beneficial to the industrial development of carbon materials from low-cost value-added byproducts from coal. Another example, Chen, Mijowska and co-workers have demon-strated the preparation of interconnected nanoporous carbon (NPC) material from direct annealing of ultra-small Al-based metal-organic complex (Al-MOC) [65]. The-obtained intercon-nected NPC exhibited the specific surface area of was 1593 m2/g, the total pore volume was 2.49 cm3/g, good electrical conductivity, led to a large specific capacitance of 205 F/g, with a voltage range from 0 to 1.2 V, in symmetric supercapacitor, and a large energy density of 10.25 Wh/kg, in an aqueous electrolyte, suggesting a large potential in supercapacitors.
2.3. Hydrothermal carbonization methodThe hydrothermal carbonization method produces a partially carbonized product, named hydrochar, which exhibits huge oxygen-functionalities and a lower condensation degree [66, 67]. The hydrochar is an appealing source for carbon yield with tailorable surface functionalities and porosity. Consequently, hydrochar from hydrothermal has poorly developed porosity with a low specific surface area. Therefore, to enhance the physical and chemical features of as-prepared materials, carbonization/activation is required [68]. Also, hierarchically porous carbon materials derived from hydrochar has a huge number of heteroatoms [69]. The presence of surface containing functional groups on different carbons electrode materials may foster capacitance in both organic and aqueous electrolytes while the electrochemically inert functional groups on the carbon surface bolster the wettability of the carbon electrode material, therefore enhancing the specific capacitance by full exploitation of surface and the ions accessibility [70].
2.4. Other methodsBesides the aforementioned preparation methods, other methods were also proposed by researchers. For the first time, Gong et al. have developed the new technique named the controlled carbonization of polymers which stands for turning the waste polymers precursors into the carbonaceous materials with well-defined morphologies and structures [71]. They have also presented the application of the prepared materials in energy storage devices and environmental remediation. Thus, the designed controlled carbonization of polymers has been certified to be a powerful strategy for preparing the carbon materials suitable for supercapacitors and other realms.
Likewise, Liu and co-workers have presented for the first time new preparation method of the three carbonization means, that is, "combined catalysis", "templating carbonization", and "fast carbonization" as well as the crucial factors of polymer carbonization and its working system [72]. The resultant carbon materials exhibited potential applications for different fields such as energy storage, catalysis, environmental remediation, and gas adsorption. Their work results demonstrate a great contribution to electrochemical energy storage device development and the scientific community.
Recently, Yang and co-workers have developed an innovative, green, and biological activated method to synthesize the activated carbon from biomass material, banana peel waste via yeast without physical or chemical activation [73]. The as-synthesized activated carbon exhibited a moderate specific surface area of 1084 m2/g and a high packing density. The maximum gravimetric specific capacitance of 476 F/g was achieved in 1 mol/L H2SO4 electrolyte. Due to the enriched pseudocapacitance sites, the YBP symmetric supercapacitor delivered a high volumetric specific capacitance and energy density of 264 F/cm3 and 23.5 Wh/L, respectively. The supercapacitor presented high cyclic stability with 94% capacitance retention for voltage values up to 1.6 V after 10, 000 potential cycles, indicating the potential application of this supercapacitor in miniature electronic devices. The developed strategy in their work is a sustainable solution for environment protection and a great contribution to energy storage devices, particularly.
To find a sustainable solution to the increase in the problem and environmental burden caused by plastic waste discarded every-where. One sustainable solution is to use the enzyme able to degrade the plastic waste. PETase is an enzyme discovered in 2016 by Shosuke Yoshida et al., and it is secreted by a plastic-munching bacterium called Ideonella sakaiensis 201-F6. The research has proved that the PETase is capable to digest the PET and then break it down into the small-and more environmentally friendly-components [74]. Later, Rey-Ting Guo and co-workers have presented the structures of a novel PETase from the PET-consuming microbe Ideonella sakaiensis in complex with substrate and product analogs [75]. They concluded that PETase hydrolyzes PET into soluble building blocks and provides an appealing avenue for the next engineering and the bioconversion of plastics waste and resulting compounds can be used as carbon precursors for preparing new electrode materials for supercapacitors at low-cost production.
3. Industrial waste-derived carbon electrode materials for supercapacitors 3.1. Classification of plastic waste for supercapacitor-scale of carbonMany years ago, the production of plastics has been increased dramatically. In 2011, the world production of plastics achieved a high record, 280 million tons, roughly two-thirds of which were contributed jointly by China (≈23%), European Union (EU-27, 21%), USA (16%) and Japan (5%), the rest of world NAFA (20%), Middle East Africa (7%), Latin America (5%), CIS (3%) [76]. As of 2015, approximately 6300 Metric tons (Mt) of plastic waste had been generated, around 9% of which had been recycled, 12% was incinerated, and 79% was accumulated in the landfills or the natural environment. If current production and waste management trends continue, roughly 12, 000 Mt of plastic waste will be in landfills or in the natural environment by 2050 [77]. How to dispose of plastics waste has brought out global attention? To turn the plastic waste and other industrial wastes into active materials for supercapacitor applications is a powerful strategy and sustainable technology for environmental protection and econom-ic development for industrialized countries. The most polymers are large-produced and low-cost; they are readily discarded after use. The percentage of carbon content in the produced plastic is as follow polyethylene (86%), polypropylene (86%), polyvinyl chloride (PVC), polystyrene (92%), polyethylene terephthalate (≈63%) and low-density polyethylene (LDPE), polyacrylonitrile (PANI) (68%). More importantly, plastic wastes are the most promising carbon precursors for preparing carbon materials due to their high carbon content, plentifulness, and as well as low-cost. Researchers have long recognized that the polyethylene in plastic bags could be a cheap precursor of energy-storing carbon due to their high carbon content.
3.1.1. Polyethylene (PE) plastic bagAs the days go on, the production of polyethylene (PE) waste increases and tough to recycle. Several years ago, PE was suggested as a cheap (1 $/kg) carbon precursor with a high carbon content (86%). Harnessing polyethylene plastic waste bags into active materials for supercapacitors is a great achievement and cost-effective strategy for environmental protection and development of renewable energy sources particularly. However, porous carbon is mostly used in supercapacitors as effective active materials owing to their high surface area, excellent conductivity, low price, tailorable size and good chemical and physical stability combined with introducing heteroatoms into the structure.
Recently, Yang et al. have proposed a cost-effective strategy for turning polyethylene waste bag into hierarchical porous activated carbon materials by using polyethylene bag waste mixed with basic magnesium carbonate pentahydrate as fire retardants and subsequently activated in ammonia (denoted as PE-C-900NH3) (Fig. 3b) and resulting materials were used for supercapacitor [61]. The PE-C-900NH3 exhibited a high specific surface area of 1219 m2/g, a large mesopore of 1.97 cm3/g, high purity, and a small ratio of O/N. The PE-C-900NH3 showed the high specific capacitance of 244 F/g at 0.2 A/g and cycling stability around 97.1% retention of its initial capacity after 10, 000 potential cycles at 2 A/g. A high energy density of 43 Wh/kg was achieved for the PE-C-900NH3 symmetrical supercapacitor at a high voltage of 4 V in EMIMBF4 electrolyte.

|
Download:
|
| Fig. 3. (a) Flow chart showing the preparation of activated carbon from waste tires as precursors. Reprinted with permission [42]. Copyright 2014, American Chemical Society. (b) Conversion of polyethylene plastic bag into PE-C-900NH3 Reprinted with permission [61]. Copyright 2019, Elsevier. (c) Schematic illustration showing the process of synthesizing PCNS from mixed plastics. Reprinted with permission [92]. Copyright 2014, American Chemical Society. | |
Later, Yang and co-workers have developed more green cost-effective technology and powerful strategy than conventional method for preparing graphene/mesoporous carbon (denoted as G@PE40-MC700) electrode materials derived from polyethylene (PE) plastic waste combined with graphene oxide (GO) additive and flame retardant via low-temperature carbonization at 700 ℃ without adding any activating agent as depicted in Fig. S8a (Supporting information) [78]. The G@PE40-MC700 presented a high surface area of 1175 m2/g and a huge amount of mesopores of 2.3 m3/g. The obtained G@PE40-MC700 exhibited the higher energy density of 63.3 Wh/kg in a high-voltage (4.0 V) symmetric supercapacitors in EMIMBF4 electrolyte with enhanced cycling stability of 89.3% after 5000 cycles (Fig. S8b in Supporting information). Their study reveals that the high capacitance and rate capability of G@PE40-MC700 were due to the synergistic effect of graphene and the mesoporous carbon composites.
3.1.2. Low-density polyethylene (LDPE)Another contribution was also made by He and co-workers, where the hierarchical porous carbon (HPC) spheres were manufactured by using LDPE waste through autogenic pressure carbonization followed by KOH activation. The as-synthesized HPC presented a micrometer-scale carbon sphere, a large specific surface area of 3059 m2/g, and more functionalities groups. The as-prepared HPC showed excellent performance of 355 F/g at 0.2 A/g in 6 mol/L KOH electrolyte, a high energy density of 9.81 Wh/kg at a power density of 450 W/kg, and outstanding cycling stability [67].
3.1.3. Plastic waste-based fluorine and chlorideThe plastic waste containing halogens like fluorine and chlorine, polyvinylidene fluoride (PVDF), polyvinylchloride (PVC) and polytetrafluoroethylene (PTFE) are also promising carbon precursors for synthesizing carbon materials for supercapacitors. PVC plastics play a great role in numerous areas, such as healthcare, packaging, and construction materials. After their use, a large amount of PVC wastes is greatly scattered in the environment and causing environmental problems. The landfills or incineration of PVC wastes are not satisfactory technology because they release other byproducts such as chlorine, hydrogen chloride, and organochlorine, which are harmful to human life and environments, hard to tackle [79, 80].
Sun and co-workers have suggested an effective and green method to convert PVC plastics into carbonaceous materials via KOH-assisted room temperature dehalogenation, followed by the formation of clean byproducts of potassium chloride and water as presented in Fig. S9a (Supporting information). The as-prepared carbon materials derived from PVC plastics waste were applied for supercapacitor. The porous carbon material derived from plastic wrap (PW-C) exhibited an excellent performance of 399 and 363 F/g at 1.0 A/g in 6.0 mol/L KOH and 1.0 mol/L H2SO4 electrolytes, respectively, (Fig. S9b in Supporting information) [81]. They concluded that the developed method is capable of treating PVC plastic wastes safely and efficiently and converting them into valued-added porous carbon electrode materials. The research group of Chang has designed a facile method of converting polyvinyl dichloride (PVDC) plastic waste into multiple doped carbon materials [82]. They have prepared N, S-doped carbon materials by employing the room-temperature dehaloge-nation of PVDC promoted by KOH combined with the different organic dopants. The prepared electrode material delivered an excellent specific capacitance of 427 F/g at 1.0 A/g in an acidic electrolyte and also maintained around 60% of capacitance at a high current density of 100.0 A/g. Zhang and co-workers [83] transformed the PTFE plastic waste into nanoporous carbon throughout the direct carbonization in the presence of zinc powder as a hard template. The obtained nanoporous carbon exhibited a large BET surface area of 800 m2/g and a high total pore volume of 1.6 cm3/g, also provided an excellent specific capacitance of 313.7 F/g at 0.5 A/g. In addition, it presented superior cycling stability with high capacitance retention of 93.10% after 5000 cycles. The nanoporous graphitic carbon materials were also prepared by using PVDF waste and Ni(NO3)2·6H2O as the graphitic catalyst [84]. From deep investigation, the authors found that the carbonization temperature plays a crucial role in determining the pore structures as well as their electrochemical performances. They concluded that the increase of the carbonization temperature from 800 ℃ to 1200 ℃, the corresponding porosity has slightly decreased, accompanied by an increase of graphitization degree. However, the air-pollution induced by carbonization of plastic waste-based fluorine and chloride should also be concerned.
3.1.4. Polystyrene (PS)Another plastic waste, polystyrene has caused serious environmental problems due to its overuse and unsatisfactory method to recycle effectively. Converting it into functional carbon materials for supercapacitor applications is one of the cost-effective ways to recycle polystyrene and other waste plastics. A straightforward and efficient method was developed for the preparation of three-dimensional (3D) network structure PC by using Friedel-Crafts reaction.
In 2018, Chen and co-workers demonstrated a facile way to fabricate nitrogen-doped porous carbon nanosheets (NPCNs) from polystyrene waste [41]. In the preparation procedure, Zn and Co bimetallic zeolitic imidazolate framework (CoZn-ZIF) nanocrystals grown on magnesium hydroxide [Mg(OH)2] sheets as a template (named Mg(OH)2@CoZn-ZIFs). At high-temperature treatment, Mg(OH)2 converts to magnesia (MgO), which helps to convert the gas-phase carbon precursors derived from PS decomposition into carbon framework. The as-prepared NPCNs with porous structure and large specific surface area were obtained. The N-PCNs exhibited a specific capacitance of 149 F/g at 0.5 A/g in 6 mol/L KOH electrolyte and excellent cycling stability of 97.6% even after 5000 cycles. Another report was done by Chen et al. where polystyrene waste foam was used for preparing a three-dimen-sional (3D) network structure PC with a facile and efficient method [85]. The prepared porous carbon displayed an excellent electrochemical capacitance of around 208 F/g at 1 A/g, and a superior energy density of 22.5 Wh/kg was achieved at a power density of 1024.4 W/kg. Meanwhile, it also displayed excellent capacitance retention at 94.3% over 5000 cycles at 5 A/g.
Very recently, another study on upcycling plastic waste dumped in the environment was presented by Min, Chen et al., where mesoporous carbon nanosheets (CNS) were prepared by mixing polystyrene waste and MgO as a template and subse-quently carbonized at 700 ℃ in an argon atmosphere for 1 h [86]. The pore structure of the-synthesized CNS was further tuned by KOH activation; the resulted hierarchical porous carbon sheets presented a specific surface area of 2650 m2/g and a pore volume of 2.43 cm3/g. The electrochemical measurement was performed in a three-electrode system; the hierarchical porous carbon sheets exhibited a specific capacitance of 323 F/g at 0.5 A/g and 222 F/g at 20 A/g in 6 mol/L KOH electrolyte, good rate capability and cycle stability (92.6% of capacitance retention after 10, 000 cycles). More importantly, an energy density of 44.1 Wh/kg was also displayed with a power density of 757.1 W/kg in an organic electrolyte. More consideration, their developed strategy presents a facile approach for turning plastic waste into high value-added products, which will potentially pave the way for the treatment of plastic waste in the future.
Later, Wen, Chen and co-workers [87] have prepared porous carbon sheets (PCSs) derived from polystyrene waste through the MgO template combined with KOH activation at 800 ℃ for 2 h under argon atmosphere. The as-prepared PCSs displayed surface area of 1163 m2/g and pore volume of 2.6 cm3/g after KOH activation, and specific capacitances of 135 and 97 F/g at 1 mV/s and at 1 A/g in 1 mol/L H2SO4 electrolyte and excellent rate performance of 82.9% retention at 20 A/g in symmetric supercapacitors were achieved. Moreover, the energy density of 3.4 Wh/kg with a high-power density of 250 W/kg in the aqueous electrolyte was achieved. Remarkably, PCS showed excellent capacitance retention of 92.4% even after 10, 000 cycles, clearly presenting the strong long-term stability.
Chen, Zhao, Tang and co-members [88] have successfully synthesized porous carbon flakes (PCFs) with the large specific surface area of 1087 m2/g, a pore volume of 4.42 cm3/g and high conductivity by direct pyrolysis of polystyrene waste through template method. After, manganese dioxide (MnO2) nanosheets were selectively deposited on the surface of resultant PCFs to form hybrid PCF-MnO2 materials. Due to the outstanding features of the prepared PCFs, native high specific capacity of MnO2, and positive synergistic interaction between PCF and MnO2, the PCF-MnO2 materials showed an ultrahigh capacitance of 308 F/g at 1 mV/s and 247 F/g at 1 A/g in LiCl electrolyte, and excellent cycle stability of 93.4% capacitance retention over 10, 000 cycles at 10 A/g in symmetric supercapacitor device.
Their work demonstrates a convenient strategy for designing cost-effective technology and high-performance electrode mate-rial for electric capacitors. More importantly, it reveals a potential way to recycle polystyrene waste into high-valued products on large-scale with disposing of polymeric waste to alleviate environmental concerns.
3.1.5. PET wasteThe treatment of PET bottles waste accumulated in the environment and ocean is a big challenge. Chen et al. have turned the PET waste into high-valuable electrode materials for electrical double supercapacitors [89]. They have established an easy strategy to efficiently convert PET beverage bottles waste into porous carbon nanosheet (PCNS) through catalytic carbonization process and KOH activation. The PCNS features an ultrahigh specific surface area of 2236 m2/g, hierarchically porous architec-ture, and a large pore volume of 3.0 cm3/g. Benefit from these physicochemical properties, the outstanding super-capacitive performance of 169 F/g in 6 mol/L KOH electrolyte and 135 F/g in 1 mol/L Na2SO4 electrolyte was reached.
Also, PCNS displayed a high specific capacitance of 121 F/g and an energy density of 30.6 Wh/kg at 0.2 A/g in 1 mol/L TEATFB/PC electrolyte. When the current density increases to 10 A/g, the capacitance remains at 95 F/g, indicating the extraordinary rate capability. Another strategy was developed to tackle PET waste, Noha A. Elessawy and co-workers have prepared the 3D sponge nitrogen-doped graphene (NG) by mixing PET waste with urea in one simple step and industrial, scalable green synthesis [47]. The as-prepared NG exhibited an outstanding performance with the specific capacitance of 405 F/g at 1 A/g, and an energy density of 68.1 Wh/kg and a high maximum power density of 558.5 W/kg in 6 mol/L KOH electrolytes were achieved from the optimized sample. The NG samples presented appropriate cyclic stability with capacitance retention of 87.7% after 5000 cycles at 4 A/g with high charge /discharge duration.
3.1.6. Mixed plastic wasteConsiderable attention was also paid to the utilization of plastics waste-derived carbon in energy storage. The team of Chen, Gong, and Tang [90] have produced porous carbon nanosheets (PCNSs) under catalytic carbonization of "real-world" mixed waste plastics on organically-modified montmorillonite (OMMT) and subsequently KOH activation. The-obtained PCNSs showed a high specific surface area of 2198 m2/g and a huge pore volume of 3.0 cm3/g. The PCNSs exhibited a superior performance with high specific capacitances approaching 207, 137 and 120 F/g at 0.2 A/g in 6 mol/L KOH, 1 mol/L Na2SO4 and 1 mol/L TEATFB/PC electrolyte, respectively. The results from their work not only provides a promising method to upcycle mixed waste plastics but also puts forward an easy cost-effective strategy for the large-scale production of porous carbon nanosheets (PCNSs) potential candidate materials for supercapacitors. The research team directed by Tang has prepared carbon nanotubes with high yields and of good quality from the mixture of PP, PE, PS and their blends carbonized via the catalyst of nanosized carbon black and Ni2O3. The as-prepared the CNT and the assembled supercapacitor exhibited a high specific capacitance as compared to super-capacitors using commercial carbon nanotubes (CNTs) and carbon black (CB) [91]. In 2014, research group of Chen and Tang have developed a novel, easy, and cost-effective strategy to convert mixed plastics of polypropylene, polyethylene, polystyrene, poly (ethylene terephthalate), and polyvinyl chloride on organically modified montmorillonite into high value-added porous carbon nanosheets (PCNS) Fig. 3c [92] through carbonization and further activated with KOH. The PCNS exhibited a high specific surface area of 1734 m2/g and a large pore volume of 2.4 cm3/g with high purity (more than 99.5%). More importantly, PCNS showed a high performance in the uptake of carbon dioxide and the storage of hydrogen. Also, the degradation products of mixed plastics such as hydrogen, propylene, and benzene during the growth of CNS could be used as important chemicals and fuels. The results from their work demonstrate that not only pave the way for large-scale utilization of mixed waste plastics but also advance the sustainable production of valuable PCNS for different applications such as energy storage.
3.2. Other industrials waste for supercapacitors-scale of carbon materials 3.2.1. Waste tiresTo date, many tires are unavoidably discarded in the environment because vehicle industries develop quickly, and therefore, their treatment is a global concern. The waste tires are not degraded easily and resist to decompose them under chemically and strong physical conditions due to its interconnected structure and possession of different additives, and long life [93]. The most waste tires are widely dumped in the environment and accumu-lated in landfills, which are not cost-effective technology to dispose of them. The sustainable solution which is economical and ecological would be to upcycle waste tires and employ them as carbon precursors for high value-added materials with potential applications as active materials in renewable energy sources and energy storage device.
Later, Wu et al. have designed an effective synthetic method for turning waste tires into activated carbon via the pyrolysis and chemical activation techniques as shown in Fig. 3a [42]. They explained that to control the activation parameters can tune multiple physical properties of the prepared activated carbon, which may influence the performance of the activated carbon electrode. From their investigation, they conclude that ion diffusion in the liquid electrolyte is the rate-limiting factor for the rate capability of activated carbon electrode. Finally, the best EDLC-type supercapacitor electrode displayed a specific capaci-tance of 106 F/g as depicted in Fig. S10 (Supporting information). In 2015, Cui and Liu contributed to the management of hydrolyzing waste tires, which causes the world to increase environmental and economic challenges. They have prepared ACs derived from the industrial pyrolytic tire char (PTC) by using a steam activation method and evaluated as promising active electrode materials for supercapacitor [58]. For the as-prepared AC-800-4 electrode presented a high specific capacitance of 190 F/g in the initial cycle and has a large decline in the first 100 cycles, which may be caused by the electrochemical oxidation of functional groups and the consumption of electrolyte. However, after 100 cycles, the specific capacitance remains almost constant (100 F/g). The AC-850-2 electrode shows similar behavior with 210 F/g at the first cycle and 90 F/g after 100 cycles. They found that activation temperature and time have a great effect on yield and structural properties. The higher content of oxygen-functionalities groups has enhanced the capacitance and boost the carbon and electrode wettability. Li and Sun's research group have treated the waste tires and for the first time, achieved to directly turn them into 3D graphene by using low-cost chemical agents and simple setup and alkaline-assisted one-step pyrolysis process [48]. The 3D graphene structure presented a high conductivity of 18.2 S/cm, and favorable hierarchical with rich porosity, and can be used as a decent energy storage material. For a supercapacitor electrode, it exhibited a high capacitance of 324.9 F/g at 0.2 A/g and excellent cyclic life (retention of 95.9% after 10, 000 cycles). Their work not only suggests a new approach for high-value reuse of waste tires but also provides a potential low-cost feedstock for large scale production of graphene.
The research group directed by M.P. Paranthaman and Y. Gogotsi has used waste tires and conducting polymer to manufacture supercapacitors [94]. They synthesized a three-dimensional meso-/microporous network with a specific surface area of 1625 m2/g. The narrow pore-size distribution and high surface area led to good charge storage capacity, especially when used as a three-dimensional nano scaffold to polymerize polyaniline (PANI). The composite paper was highly flexible, conductive, and showed capacitance of 480 F/g at 1 mV/s with excellent capacitance retention of up to 98% after 10, 000 charges/ discharge cycles. The high capacitance and long cycle life were attributed to the short diffusional paths, uniform PANI coating, and tight confinement of the PANI in the inner pores of the waste tire-derived carbon through π-π interactions, which minimized the degradation of the PANI upon cycling. They concluded that PANI/TC or a combination of TC with other redoxactive polymers and metal oxides might find its application in the large-scale energy-storage systems, where materials with moderate cost and high energy density are critically needed.
3.2.2. Printed circuit board (PCB) wasteThe fast-growing of electronic wastes (e-wastes) produces approximately 20-50 million tons per year, and their increase rate is between 4-5 percent annually [95, 96]. Particularly, the printed circuit boards (PCBs) are found in almost every electrical and electronic gadget. PCB is a complex structure to assemble and also to disassemble; the PCBs are built by using metals (such as Cu, Al, Fe, Sn, Sb, Pb) and non-metals (for example thermosetting resins, reinforcing materials, brominated flame retardants (BFRs) [97]. PCBs are categorized into single/double-sided and multi-layered PCB. The PCBs in computers and communication equipment are made from glass fiber reinforced epoxy resin (referred to FR-4 type), but televisions, monitors, and home electronics predomi-nantly use PCBs made of phenolic based polymeric material (FR-2 type). Additionally, in PCBs, the arrangement of polymers (polyethylene, propylene, polyesters, etc.), ceramics (mainly, SiO2, Al2O3) and metals assembled and manufactured in one substrate. Waste PCB of a monitor from an end of life computer is considered to be polymer-rich based material (FR-2) produces carbon-rich nonmetallic residue after the pyrolysis process. Therefore, this alternative secondary waste non-metallic fraction (NMF) can be considered for replacing the conventional sources to produce activated carbon, which helps in maintaining much-needed sustainability. For instance, Rajarao et al. reported the activated carbons materials derived from non-metallic fractions from PCBs waste throughout physical activation after pyrolysis processes as shown in Fig. S11a (supporting information) [47]. The prepared activated carbon materials exhibited the highest surface area of 700 m2/g and a higher volume of 0.022 cm3/g annealed at 850 ℃ for a 5 h and was electrochemically evaluated (Fig. S11b in Supporting information). The synthesized activated carbons showed a specific capacitance of 220 F/g at 30 mV/s and 156 F/g, even at 100 mV/s with the retention value of 98% over 1000 cycles. They have proposed an effective approach for producing value-added activated carbon materials from PCB waste demonstrating electrochemical behavior promising for the energy storage realm.
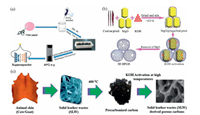
3.2.3. Waste filter paperFor laboratory experiments, the filter papers are thrown away or kept in the trash can or discarded in the environment or sometimes burned after filtration, which generates hazards compounds. For safety and environment issues, there is a need for a sustainable approach to dispose of waste filter paper. The research group of Chang and Yang has prepared hierarchical activated porous carbon (APC) from recycled waste filter paper via convenient chemical activation with ZnCl2 (Fig. 4a) [60]. The produced activated carbon presented a large surface area of 2170 m2/g, suitable pore size, and prominent porosity. The as-synthesized APC showed a specific capacitance of 302.3 F/g in 6 mol/L KOH at 1 A/g. Furthermore, the APC electrode exhibited good long-term cycling stability, and 95.4% of its initial specific capacitance at 5 A/g and was maintained even after 10, 000 cycles. From the presented results, the suggested method offers a new view of exploring waste products to develop high-performance activated carbon electrode materials for ideal applications in electric doublelayer capacitors.
|
Download:
|
| Fig. 4. (a) Schematic diagram of the preparation of APC-x-materials from recycled waste filter paper and application in a supercapacitor. Reproduced with permission [60]. Copyright 2015, The Royal Society of Chemistry. (b) The direct fabrication process of 3D HPGB from the coal tar pitch (CTP). Reproduced with permission [100]. Copyright 2014, The Royal Society of Chemistry. (c) Flow chart of converting solid leather waste into porous carbon materials. Reprinted with permission [102]. Copyright 2017, Elsevier B.V. | |
3.2.4. Compact disc waste
The compact discs (CDs) or digital versatile discs (DVDs) are portable storage for recording, storing, and playing audio, video or other digital data. In 2003, accounting for the production of CDs and DVDs worldwide was 12 billion pieces [98] and has highly grown. Waste CDs have contributed highly to dump because of the complex nature of the manufacturing process (approximately 10% of all made CDs are disqualified), postconsumer CDs, and destruction of CDs due to the artist's right issue (unsold copies). In 2018, Rifat Farzana and team-mates have prepared the microporous activated carbon from compact discs waste via physical activation method for supercapacitor applications [99]. The as-synthesized activated carbon showed the highest specific surface area of 1214 m2/g achieved at 900 ℃ and 8 h for activation. The produced activated carbon displayed good specific capaci-tance, cycle stability and good rate ability where the specific capacitance 51 F/g at the current density of 10 mV/s. The energy density of 21.43 Wh/kg at power density 0.7 W/kg and 9.68 Wh/kg at power density 2.2 W/kg were also achieved in aqueous electrolytes. Therefore, this innovative strategy of exploiting outdated CD activated carbon as supercapacitor electrode mini-mizes the environmental issues of waste CDs and offer an alternative active electrode material for supercapacitor application.
3.2.5. Coal tar pitch (CTP)Coal tar is a low-cost and plentiful waste produced during the coke production process. The annual output of coal tar is around 20.0 million tons in China only. Coal tar is rich in polycyclic aromatic hydrocarbons that are active and linked by the single chain or bridge bonds. Compared with the carbon connection in graphene, the polycyclic aromatic hydrocarbons in coal tar are facile aromatized to make large sheet-like films that can be further converted into graphene-like nanosheets. Coal tar pitch (CTP), the main residue from the distillation of coal tar (more than 55%), is cheap and abundant with plasticity characteristics. There are many polycyclic aromatic hydrocarbon molecules in CTP, which are connected by the single chain or bridge bonds. Taking into account these chemical compositions; thus, CTP is regarded as carbon precursors for preparing carbon materials for supercapacitor. In 2014, He and co-authors developed 3D hollow porous graphene balls (HPGBs) from the coal tar pitch for the first time by a simple nano-MgO template strategy coupled with KOH activation (Fig. 4b) [100]. The as-made HPGBs feature a 3D spherical architecture with a thin porous shell that has a high specific surface area and consists of macropores, mesopores, and micropores in a well-balanced ratio.
The as-synthesized HPGBs electrode for supercapacitor dem-onstrated a high specific capacitance of 321 F/g at 0.05 A/g, an excellent rate performance of 244 F/g at 20 A/g, and good cycle stability over 94.4% capacitance retention after 1000 cycles. The good electrochemical performance of the prepared materials is due to the networks with a balanced pore distribution in the shells of HPGBs support to enhance the conductivity of electrons, and to decrease the resistance to ion transfer owing to the more porous channels and active sites in the thin carbon shells permit for storage and fast transportation of electrolyte ions. Their work results may provide a new approach for effective and mass production of affordable price spherical graphene, aromatic hydrocarbon sources such as coal tar and heavy oils, for super-capacitors applications. He and co-workers have developed a layered-hydroxide-template strategy combined with in-situ chemical activation to prepare porous carbon nanosheets (PCNSs) from coal tar [101]. The as-synthesized hierarchical PCNSs constituted by large sheets possess high electric conductivity and plentiful of short pores for rapid ion transport and adsorption. The as-prepared PCNS electrodes exhibited a high capacitance of 296.2 F/g at 0.05 A/g in 6 mol/L KOH electrolyte, excellent rate performance with capacitance remaining at 220.7 F/g at 20 A/g and cycle stability with more than 97.2% of initial capacitance after 11, 000 cycles.
3.2.6. Solid leather wastes (SLW)The solid leather wastes are byproducts produced during the leather crusting process. The solid leather wastes are susceptible to serve as the porous carbons precursor material due to their abundance in the environment and low-cost. To convert the solid leather wastes into porous carbon materials for supercapacitor applications is a great achievement on the industrial scale and economy of the country in particular.
Kennedy and team-workers synthesized porous carbons from solid leather wastes (SLW) by using carbonization and chemical activation and were served as the active electrode material for supercapacitors in H2SO4 medium as shown in Fig. 4c [102]. Electrochemical measurements reveal that porous carbon activated at 900 ℃ demonstrated a maximum capacitance of 1833 F/g at 1 mV/s in 1 mol/L H2SO4 electrolyte. Besides, the produced porous carbon electrodes exhibited excellent cycling stability after 500 cycles at 20 A/g. To conclude, the results from their work proposed that prepared carbon active materials from Solid Leather Wastes can serve as desirable candidate materials for supercapacitors.
3.2.7. Sodium lignosulfonate wasteZhang and team-members from the Research Institute of Chemical Defense (Beijing, China) have successfully synthesized an interconnected hierarchical porous carbon from sodium lignosul-fonate industrial waste via direct carbonization without adding templating and activating agents [103]. The as-synthesized carbon electrode material presented a moderate specific surface area of 903 m2/g and high contents of 8.11 at% oxygen and 1.76 at% nitrogen, which could enrich the electrolyte-affinitive surface area in an aqueous electrolyte. The electrochemical evaluation of the prepared carbon electrode material for symmetric supercapacitor showed a high gravimetric capacitance of 247 F/g, a volumetric capacitance of 240 F/cm3, and areal capacitance of 27.4 μF/cm2 at 0.05 A/ in 7 mol/L KOH electrolytes. Furthermore, a superior energy density of 8.4 Wh/L at 13.9 W/L and a power density of 5573.1 W/L at 3.5 Wh/L, as well as excellent cycling stability after 20, 000 cycles at two various current densities were reached for the assembled supercapacitors. All results of their work suggested a powerful and environment-begin method favorable for mass-production of nanoporous carbons by employing plentiful and sustainable industry waste sodium lignosulfonate as carbon materials precursors, demonstrating potential applications in high-performance super capacitors. Wang and co-workers have synthesized the nitrogen-doped porous carbon derived from sodium alginate and urea via one-pot annealing at 700 ℃. The as-synthesized materials showed a mesoporous and macroporous structure and delivered the specific capacitance of 180.2 F/g at 1.0 A/g with excellent cycling life with 96% capacitance retention after 5000 potential cycles in 6 mol/L KOH electrolyte [104]. The result from their work shows the potential application of industrial waste for preparing electrode materials for supercapacitor applications.
3.2.8. Biobased ligninIn 2019, Magdalena Titirici and team-members have reported the preparation of the ordered mesoporous carbons (OMCs) produced from lignin via the evaporation induced self-assembly (EISA) method. They have proved that it is possible to substitute half of the phloroglucinol (a well-known carbon precursor currently not derived from bio-precursors) by hardwood organosolv lignin while obtaining a highly ordered pore structure. Notably, they have also used glyoxal instead of formaldehyde as a crosslinker, which makes the synthesis route even "greener" concerning the toxicity of the precursors and their renewable sourcing. The as-prepared bio-based material attains a volumetric energy density of 3 Wh/L at 1 kW/L, which could be easily improved by adapting the microporous structure to the electrolyte ion size [105]. Many efforts were devoted to turning the industrials waste, plastic waste and other waste into electrode materials for supercapacitors application, but we can say it is the beginning of a journey. Thus more research is needed to contribute to shaping and protecting the environment without losing any materials.
4. Conclusions and outlooksAlthough active electrode materials from coconut shells are excellent supercapacitor materials for high-rates and high-power widespread applications. Some challenges and issues remain. The plastic waste and other industrial waste materials are abundant, cheaper, and even dumped in the environment due to the rapid growth of the industry. Therefore, to turn them into functional carbon materials for the energy storage device as promising electrode materials needed for storing energy in the device is a paramount achievement in the science and scientific community. For future energy storage systems, supercapacitor should be able to store and produce energy at very high-rates and support many charges and discharge cycles.
(1) A new synthetic method for activated carbon. To design the environmental method for transforming low-cost plastic waste into carbon materials with high electrocapacitive features compared with commercial activated carbon from coconut shell will reduce the cost of production. To reduce the cost of production compared to 4 $/kg of traditional activated carbon from coconut shell will be a great achievement for introducing the new electrode material in the supercapacitor market. To develop a new electrode with an energy density which is more than double that of activated carbon from coconut shell, it will create the carbon market. The energy density of new electrode material should be raised via an appropriate choice of electrolyte with a high potential range (i.e., ionic liquid, up to 4 V) and also building the hybrid, which combines capacitive and pseudocapacitive. The structure of the new electrode should be in a tailorable structure for being integrated into widespread applications such as fabricating flexible supercapacitors, solid-state supercapacitors, which is a very new technology for wearable and portable electronics promising electrochemical energy storage device. The market growth of supercapacitors is generally impeded by low energy density and cost with the latter more enhancement is necessary. The development of active materials from low-cost plastic waste and other industrial waste is stepped forward for maintaining the supercapacitor market segment.
(2) High voltage supercapacitor with enhanced energy density. To design the higher electrochemical durability at the electrode/ electrolyte interface is a crucial matter. The high working voltage range for supercapacitors is an effective method for achieving high energy density. The organic electrolyte is chemical stability, low viscosity and high conductivity which are beneficial to the active electrode materials for supercapacitor and have high voltage (2.5-2.8 V) while ionic liquid-based electrolytes (up to 4 V), thus improved the energy density of supercapacitor is achieved.
(3) The high energy density of hybrid capacitors. The hybrid supercapacitors combine an electrode with a high amount of pseudocapacitance with an electrode with a high amount of doublelayer capacitance. The faradaic pseudocapacitance elec-trode with their higher capacitance provides high specific energy while the non-faradaic EDLC electrode enables high specific power. They have higher specific capacitance value as well as their higher-rated voltage (2.2-3.8 V) and correspondingly their higher specific energy compared with symmetrical EDLC's. To develop electrodes for new hybrid-type supercapacitor electrodes as for lithiumion capacitors and battery-type capacitor leads to the high energy density. Together with a carbon EDLC electrode in an asymmetric construction offers higher specific energy than conventional supercapacitors with higher specific power, longer cycle life and faster charging and recharging times than batteries. The benefit of this type of supercapacitors is their higher voltage (1.5-4.2 V) and higher specific energy (up to 10-20 W h/kg).
(4) Flexible supercapacitor for the coming wearable electronic devices. The flexible supercapacitors are regarded as one of the most desirable energy storage devices due to their high power density, long lifespan, and sufficient energy density. For extending the applications of active material prepared from industrial waste materials, used in assembling all-solid-state supercapacitors (ASSSCs), which are lightweight, flexible, and portable, therefore, demonstrating high potential to be applied for power sources for flexible portables electronics.
(5) Supercapacitor for hybrid electric vehicles and trolleybus. The supercapacitors are used for powering transportation in cities, particularly in China. For electric vehicles and buses, charging takes place at stops when passengers are getting on or off and a 10-second full charge is reported to give a travel distance of about 5 km. Concerning stop-go hybrid system, the engine is turned off and on when the vehicle or bus stops and the accessory loads are to be met from the electric energy storage. The energy storage is recharged from regenerative braking and an engine-powered alternator or generator. Additionally, charging and discharging for bus and hybrid electric vehicles is very frequent, can reach hundreds of times a day, so often this kind of braking, energy recovery, and auxiliary output when starting, fuel-saving efficiency is very high. According to the aggregate data, the fuel-saving rate is generally between 35% and 60%, among which plug-in hybrid electric vehicle has the highest fuel saving rate, and the fuel-saving rate is more than 60%. Besides, there are two main applications of supercapacitors in buses: one is the energy recovery when the vehicle brakes, the other is the rapid energy release when the vehicle starts. We believe that the supercapacitor has a bright future and prominent for some specific applications, especially in transportations.
Declaration of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21975025, 21575015, 21203008), the Beijing Natural Science Foundation (No. 2172051), the National Key Research and Development Program of China "New Energy Project for Electric Vehicle" (No. 2016YFB0100204), State Key Laboratory for Modification of Chemical Fibers and Polymers Materials, Donghua University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.01.003.
| [1] |
M.F. El-Kady, V. Strong, S. Dubin, R.B. Kaner, Science 335 (2012) 1326-1330. DOI:10.1126/science.1216744 |
| [2] |
P. Simon, Y. Gogotsi, Nat. Mater. 7 (2008) 845-854. DOI:10.1038/nmat2297 |
| [3] |
G.P. Wang, L. Zhang, J.J. Zhang, Chem. Soc. Rev. 41 (2012) 797-828. DOI:10.1039/C1CS15060J |
| [4] |
P. Simon, Y. Gogotsi, B. Dunn, Science 343 (2014) 1210-1211. DOI:10.1126/science.1249625 |
| [5] |
Z. Yu, L. Tetard, L. Zhai, J. Thomas, Energy Environ. Sci. 8 (2015) 702-730. |
| [6] |
K. Naoi, S. Ishimoto, J.I. Miyamoto, W. Naoi, Energy Environ. Sci. 5 (2012) 9363-9373. DOI:10.1039/c2ee21675b |
| [7] |
L.L. Zhang, X. Zhao, Chem. Soc. Rev. 38 (2009) 2520-2531. DOI:10.1039/b813846j |
| [8] |
H. Helmholtz, Ann. Phys. 89 (1853) 211-233. |
| [9] |
M.S. Park, Y.G. Lim, J.H. Kim, et al., Adv. Energy Mater. 1 (2011) 1002-1006. DOI:10.1002/aenm.201100270 |
| [10] |
L.G. Gouy, J. Phys.Theor.Appl. 9 (1910) 45-468. |
| [11] |
O. Stern, Z. Elektromie, Angew. Phys. Chem. 30 (1924) 508-516. |
| [12] |
V. Aravindan, J. Gnanaraj, Y.S. Lee, S. Madhavi, Chem. Rev. 114 (2014) 11619-11635. DOI:10.1021/cr5000915 |
| [13] |
J. Balamurugan, T.T. Nguyen, V. Aravindan, N.H. Kim, J.H. Lee, Adv. Funct. Mater. 28 (2018) 1804663. DOI:10.1002/adfm.201804663 |
| [14] |
Q. Rong, W. Lei, J. Huang, M. Liu, Adv. Energy Mater. 8 (2018) 1801967. DOI:10.1002/aenm.201801967 |
| [15] |
X.J. Wang, L.L. Liu, Z.Q. Niu, Mater. Chem. Front. 3 (2019) 1265-1279. DOI:10.1039/C9QM00062C |
| [16] |
S. Repp, E. Harputlu, S. Gurgen, et al., Nanoscale 10 (2018) 1877-1884. DOI:10.1039/C7NR08190A |
| [17] |
Z. Lin, E. Goikolea, A. Balducci, et al., Mater. Today 21 (2018) 419-436. DOI:10.1016/j.mattod.2018.01.035 |
| [18] |
K. Naoi, P. Simon, Interface 17 (2008) 34-37. |
| [19] |
T. Brousse, M. Toupin, R. Dugas, et al., J. Electrochem. Soc. 153 (2006) A2171-A2180. DOI:10.1149/1.2352197 |
| [20] |
A. Rudge, I. Raistrick, S. Gottesfeld, J.P. Ferraris, J. Power Sources 47 (1994) 89-107. DOI:10.1016/0378-7753(94)80053-7 |
| [21] |
X. Qin, S. Durbach, G.T. Wu, Carbon 42 (2004) 451-453. DOI:10.1016/j.carbon.2003.11.012 |
| [22] |
V. Gupta, N. Miura, Electrochim. Acta 52 (2006) 1721-1726. DOI:10.1016/j.electacta.2006.01.074 |
| [23] |
Y.G. Wang, Y.Y. Xia, Adv. Mater. 25 (2013) 5336-5342. DOI:10.1002/adma.201301932 |
| [24] |
Z.H. Wen, X.C. Wang, S. Mao, et al., Adv.Mater. 24 (2012) 5160-5616. |
| [25] |
K. Naoi, W. Naoi, S. Aoyagi, J.I. Miyamoto, T. Kamino, Acc. Chem. Res. 5 (2013) 1075-1083. |
| [26] |
A.G. Pandlofo, A.F. Hollenkamp, J. Power Sources 157 (2006) 11-27. DOI:10.1016/j.jpowsour.2006.02.065 |
| [27] |
G.G. Amatucci, F. Badway, A. Du Pasquier, T. Zheng, J. Electrochem. Soc. 148 (2001) A930-A939. DOI:10.1149/1.1383553 |
| [28] |
K. Naoi, Fuel Cells 10 (2010) 825-833. DOI:10.1002/fuce.201000041 |
| [29] |
M. Beidaghi, Y. Gogotsi, Energy Environ. Sci. 7 (2014) 867-884. DOI:10.1039/c3ee43526a |
| [30] |
A. Yoshino, T. Tsubata, M. Shimoyamada, et al., J. Electrochem. Soc. 151 (2004) A2180-A2182. DOI:10.1149/1.1813671 |
| [31] |
W.L. Zhang, J. Wang, D.B. Ruan, et al., Chem. Eng. J. 373 (2019) 1012-1019. DOI:10.1016/j.cej.2019.05.039 |
| [32] |
F. Béguin, V. Presser, A. Balducci, E. Frackowiak, Adv. Mater. 26 (2014) 2219-2251. DOI:10.1002/adma.201304137 |
| [33] |
L.P. Yu, G.Z. Chen, Front. Chem. 7 (2019) 272. DOI:10.3389/fchem.2019.00272 |
| [34] |
K.S.W. Sing, D.H. Everett, R.A.V. Haul, et al., Pure Appl. Chem. 57 (1985) 603-619. DOI:10.1351/pac198557040603 |
| [35] |
J.S. Huang, B.G. Sumpter, V. Meunier, Angew. Chem. Int. Ed. 47 (2008) 520-524. DOI:10.1002/anie.200703864 |
| [36] |
J.S. Huang, B.G. Sumpter, V. Meunier, Chem.-Eur. J. 14 (2008) 6614-6626. DOI:10.1002/chem.200800639 |
| [37] |
Y. Gogotsi, V. Presser, Carbon Nanomaterials, 2nd ed., CRC Press, Boca Raton, 2006.
|
| [38] |
L.L. Zhang, Y. Gu, X. Zhao, J. Mater. Chem. A 1 (2013) 9395-9408. |
| [39] |
L. Weinstein, R. Dash, Mater. Today 16 (2013) 356-357. DOI:10.1016/j.mattod.2013.09.005 |
| [40] |
M. Okan, H.M. Aydin, M. Barsbay, J. Chem. Technol. Biotechnol. 94 (2019) 8-21. DOI:10.1002/jctb.5778 |
| [41] |
G.X. Wang, L. Liu, L.L. Zhang, et al., J. Electronic Mater. 47 (2018) 5816-5824. DOI:10.1007/s11664-018-6497-x |
| [42] |
M.J. Zhi, F. Yang, F.K. Meng, et al., ACS Chem. Eng. 2 (2014) 1592-1598. DOI:10.1021/sc500336h |
| [43] |
P.P. Zhao, Y. Han, X.T. Dong, C. Zhang, S.X. Liu, ECS J. Solid State Sci. Technol. 4 (2015) M35-M40. DOI:10.1149/2.0271505jss |
| [44] |
H. Shi, Electrochim. Acta 41 (1996) 1633-1639. DOI:10.1016/0013-4686(95)00416-5 |
| [45] |
H. Jiang, P.S. Lee, C. Li, Energy Environ. Sci. 6 (2013) 41-53. DOI:10.1039/C2EE23284G |
| [46] |
M. Sevilla, R. Mokaya, Energy Environ. Sci. 7 (2014) 1250-1280. DOI:10.1039/C3EE43525C |
| [47] |
R.R. Rajagopal, L.S. Aravinda, R. Rajarao, et al., Electrochim. Acta 211 (2016) 488-498. DOI:10.1016/j.electacta.2016.06.077 |
| [48] |
C. Wang, D. Li, T. Zhai, et al., Energy Storage Mater. 23 (2019) 499-507. DOI:10.1016/j.ensm.2019.04.014 |
| [49] |
L. Sun, C.L. Wang, Y. Zhou, X. Zhang, J.S. Qiu, J. Appl. Electrochem. 44 (2014) 309-316. DOI:10.1007/s10800-013-0636-0 |
| [50] |
T. Otowa, R. Tanibata, M. Itoh, Gas Sep. Purif. 7 (1993) 241-245. DOI:10.1016/0950-4214(93)80024-Q |
| [51] |
D. Lozano-Castelló, J.M. Calo, D. Cazorla-Amorós, A. Linares-Solano, Carbon 45 (2007) 2529-2536. DOI:10.1016/j.carbon.2007.08.021 |
| [52] |
E. Raymundo-Piñero, P. Azaïs, T. Cacciaguerra, et al., Carbon 43 (2005) 786-795. DOI:10.1016/j.carbon.2004.11.005 |
| [53] |
W. Qiao, S.H. Yoon, I. Mochida, Energy Fuels 20 (2006) 1680-1684. DOI:10.1021/ef050313l |
| [54] |
H. Wang, Q. Gao, J. Hu, J. Am. Chem. Soc. 131 (2009) 7016-7022. DOI:10.1021/ja8083225 |
| [55] |
A. Świątkowski, Studies in surface science and catalysis, in: A. Dąbrowski (Ed.), Adsorption and Its Applications in Industry and Environmental Protection, Elsevier Science B.V., 1999, pp. 69-94.
|
| [56] |
J. Romanos, M. Beckner, T. Rash, et al., Nanotechnology 23 (2012) 015401. DOI:10.1088/0957-4484/23/1/015401 |
| [57] |
J. Wang, S. Kaskel, J. Mater.Chem. 22 (2012) 23710-23725. DOI:10.1039/c2jm34066f |
| [58] |
X.J. He, R.C. Li, J.S. Qiu, et al., Carbon 50 (2012) 4911-4921. DOI:10.1016/j.carbon.2012.06.020 |
| [59] |
H. Zhang, X.L. Zhou, L.M. Shao, F. Lu, P.J. He, ACS Sustainable Chem. Eng. 7 (2019) 3801-3810. DOI:10.1021/acssuschemeng.8b04539 |
| [60] |
B.B. Chang, Y.Z. Guo, Y.C. Li, B.C. Yang, RSC Adv. 5 (2015) 72019-72027. DOI:10.1039/C5RA12651G |
| [61] |
Y.M. Lian, M. Ni, Z.H. Huang, et al., Chem. Engin. J. 366 (2019) 313-320. DOI:10.1016/j.cej.2019.02.063 |
| [62] |
P.W. Yu, R.J. Chen, Q. Zhang, et al., Sci. Bull. 63 (2018) 213-215. DOI:10.1016/j.scib.2018.01.012 |
| [63] |
P. Abudu, L.X. Wang, M.J. Xu, et al., Chem. Phys. Lett. 702 (2018) 1-7. DOI:10.1016/j.cplett.2018.04.055 |
| [64] |
T.T. Guan, K.X. Li, J.H. Zhao, et al., J. Mater. Chem. A 5 (2017) 15869-15878. DOI:10.1039/C7TA02966G |
| [65] |
X.Z. Shi, S. Zhang, X.C. Chen, E. Mijowska, Nanomaterials 9 (2019) 601. DOI:10.3390/nano9040601 |
| [66] |
A. Jain, R. Balasubramanian, M. Srinivasan, Chem. Eng. J. 283 (2016) 789-805. DOI:10.1016/j.cej.2015.08.014 |
| [67] |
N.A. Elessawy, El Nady J., W. Wazeer, A.B. Kashyout, Sci. Rep. 9 (2019) 1129-1138. DOI:10.1038/s41598-018-37369-x |
| [68] |
J. Jiang, J.H. Zhu, W. Ai, et al., Energy Environ. Sci. 7 (2014) 2670-2679. DOI:10.1039/C4EE00602J |
| [69] |
M. Sevilla, A.B. Fuertes, R. Mokaya, Energy Environ. Sci. 4 (2011) 1400-1410. DOI:10.1039/c0ee00347f |
| [70] |
W.T. Gu, M. Sevilla, A. Magasinski, A.B. Fuertes, G. Yushin, Energy Environ. Sci. 6 (2013) 2465-2476. DOI:10.1039/c3ee41182f |
| [71] |
J. Gong, X.C. Chen, T. Tang, Progress Polym. Sci. 94 (2019) 1-32. DOI:10.1016/j.progpolymsci.2019.04.001 |
| [72] |
J. Gong, X. Chen, X. Wen, et al., Sci. Sin. Chim. 48 (2019) 829-843. |
| [73] |
Y.M. Lian, M. Ni, L. Zhou, R.J. Chen, W. Yang, Chem.-A Eur.J. 24 (2018) 18068-18074. DOI:10.1002/chem.201803836 |
| [74] |
S. Yoshida, K. Hiraga, T. Takehana, et al., Science 351 (2016) 1196-1199. DOI:10.1126/science.aad6359 |
| [75] |
X. Han, W.D. Liu, J.W. Huang, et al., Nat. Commun. 8 (2017) 2106. DOI:10.1038/s41467-017-02255-z |
| [76] |
C.W. Zhuo, Y.A. Levendis, J. Appl. Polym. Sci. 131 (2014) 39931. |
| [77] |
R. Geyer, J.R. Jambeck, K.L. Law, Sci. Adv. 3 (2017) 1-5. |
| [78] |
Y.M. Lian, W. Utetiwabo, Y. Zhou, et al., J. Colloid Interface Sci. 557 (2019) 55-64. DOI:10.1016/j.jcis.2019.09.003 |
| [79] |
X.Y. Chen, L.X. Cheng, X. Deng, et al., J. Ind. Eng. Chem. Res. 53 (2014) 6990-6997. DOI:10.1021/ie500685s |
| [80] |
M.A. Keane, ChemSusChem 2 (2009) 207-214. DOI:10.1002/cssc.200900001 |
| [81] |
Y.N. Chang, Y.C. Pang, Q.D. Dang, et al., ACSAppl. Energy Mater. 1 (2018) 5685-5693. |
| [82] |
Y.N. Chang, G.X. Zhang, B. Han, et al., ACS Appl. Mater. Interfaces 9 (2017) 29753-29759. DOI:10.1021/acsami.7b08181 |
| [83] |
X.Y. Chen, L.X. Cheng, X. Deng, L. Zhang, Z.J. Zhang, Ind. Eng. Chem. Res. 53 (2014) 6990-6997. DOI:10.1021/ie500685s |
| [84] |
L.X. Cheng, Y.Q. Zhu, X.Y. Chen, Z.J. Zhang, Ind. Eng. Chem. Res. 54 (2015) 9948-9955. DOI:10.1021/acs.iecr.5b02490 |
| [85] |
Y.X. Zhang, Z.M. Shen, Y.F. Yu, et al., J. Mater. Sci. 53 (2018) 12115-12122. DOI:10.1007/s10853-018-2513-z |
| [86] |
C. Ma, X.G. Liu, J.K. Min, et al., Nanotechnology 31 (2020) 035402. DOI:10.1088/1361-6528/ab475f |
| [87] |
Y.L. Wen, X. Wen, K. Wenelska, X.C. Chen, E. Mijowska, Diamond Related Mater. 95 (2019) 5-13. DOI:10.1016/j.diamond.2019.03.015 |
| [88] |
J.K. Min, S. Zhang, J.X. Li, et al., Waste Manag. 85 (2019) 333-340. |
| [89] |
Y.L. Wen, K. Kierzek, J.K. Min, et al., J. Appl. Polym. Sci. 137 (2019) 48338. |
| [90] |
Y.L. Wen, K. Kierzek, X.C. Chen, et al., Waste Manag. 87 (2019) 691-700. DOI:10.1016/j.wasman.2019.03.006 |
| [91] |
X. Wen, X.C. Chen, N.N. Tian, et al., Environ. Sci. Technol. 48 (2014) 4048-4055. |
| [92] |
J. Gong, B. Michalkiewicz, X.C. Chen, et al., ACS Sustainable Chem. Eng. 2 (2014) 2837-2844. |
| [93] |
A. Quek, R. Balasubramanian, J. Anal. Appl. Pyrolysis 101 (2013) 1-16. DOI:10.1016/j.jaap.2013.02.016 |
| [94] |
M. Boota, M.P. Paranthaman, A.K. Naskar, et al., ChemSusChem 8 (2015) 3576-3581. DOI:10.1002/cssc.201500866 |
| [95] |
R. Rajarao, V. Sahajwalla, R. Cayumil, M. Park, R. Khanna, Proc. Environ. Sci. 21 (2014) 33-41. DOI:10.1016/j.proenv.2014.09.005 |
| [96] |
I.O. Ogunniyi, M.K.G. Vermaak, D.R. Groot, Waste Manag. 29 (2009) 2140-2146. DOI:10.1016/j.wasman.2009.03.004 |
| [97] |
Y.H. Ke, E.T. Yang, X. Liu, C.L. Liu, W.S. Dong, New Carbon Mater. 28 (2013) 108-114. DOI:10.1016/S1872-5805(13)60069-4 |
| [98] |
R. Zevenhoven, L. Saeed, Automotive Shredder Residue (ASR) and Compact Disc (CD) Waste: Options for Recovery of Materials and Energy, Helsinki University of Technology, Espoo (Finland), 2003 p. 74.
|
| [99] |
R. Farzana, R. Rajarao, B.R. Bhat, V. Sahajwalla, J. Industrial Engin. Chem. 65 (2018) 387-396. DOI:10.1016/j.jiec.2018.05.011 |
| [100] |
X.J. He, H.B. Zhang, H. Zhang, et al., J. Mater. Chem. A 2 (2014) 19633-19640. DOI:10.1039/C4TA03323J |
| [101] |
X.J. He, H. Ma, J.X. Wang, et al., J. Power Sources 357 (2017) 41-46. DOI:10.1016/j.jpowsour.2017.04.108 |
| [102] |
N. Konikkara, L.J. Kennedy, Mater. Lett. 205 (2017) 56-61. DOI:10.1016/j.matlet.2017.06.048 |
| [103] |
J. Pang, W.F. Zhang, J.L. Zhang, et al., Green Chem. 19 (2017) 3916-3926. DOI:10.1039/C7GC01434A |
| [104] |
C.J. Xuan, Z.K. Peng, J. Wang, et al., Chin. Chem. Lett. 28 (2017) 2227-2230. DOI:10.1016/j.cclet.2017.09.009 |
| [105] |
S. Herou, M.C. Ribadeneyra, R. Madhu, et al., Green Chem. 21 (2019) 550-559. DOI:10.1039/C8GC03497D |
 2020, Vol. 31
2020, Vol. 31 

