b Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China;
c Ningbo Research Institute, Zhejiang University, Ningbo 315100, China
Emissions of carbon dioxide (CO2) from fossil fuel combustion during industrial processes are the main cause of global warming. In this regard, electrochemical CO2 reduction to generate useful chemical fuels represents a significant way to decrease its atmospheric concentration [1]. However, it still suffers from the challenges of large catalytic overpotential required to activate CO2 molecule, and low catalytic selectivity owing to the occurrence of competitive hydrogen evolution reaction (HER) in electrolyte [2].
In recent years, a wide variety of metal based catalysts (such as Ni [1f], Sn [3], In [4] and Cu [5]) have been explored to promote electrocatalytic CO2 reduction reaction due to their superior catalytic performance and robust stability. However, the reduced products of electrocatalytic CO2 reduction (CO2ER) for above mentioned metal based materials are quite different. The noble metals and Ni based catalysts can usually selectively reduce the CO2 into producing CO product [6], whereas the Sn and In based catalysts exhibit excellent catalytic selectivity for formate production [3, 4], while Cu based catalysts could achieve the transformation of CO2 into producing hydrocarbon and alcohol [5]. Among all the reduced products from CO2ER process, formate is easier to be yielded as liquid-phase product as compared to alcohol, since its lower theoretic potential and less reaction steps of proton coupled electron transfer process [7].
Recently, metal/metal oxide derived electrocatalysts have been displayed to boost catalytic activity for CO2ER with respect to their fully reduced counterparts [1a, 8]. For instance, a Sn/SnOx electrode exhibited greatly enhanced CO2ER activity relative to a typical Sn electrode [8c]. It was reported that partial oxidation of the cobalt atomic could effectively increase their catalytic activity towards formate production relative to typical cobalt atomic layers [8a]. Obviously, the combination of undercoordinated sites from partial reduction of metal oxide, and the synergetic effect between metal oxide and metal can play an important role in boosting CO2ER selectivity [8b]. Bismuth (Bi) composites have been widely reported to deliver high selectivity and catalytic activity for CO2ER, crediting their strong adsorption of CO2·- intermediates [9]. However, some key problems still remain, such as low current density and poor catalytic stability [9c, 10]. It is thus quite vital to further probe into the feasibility of preparing Bi based catalysts with good catalytic activity and selectivity.
Herein, we developed a compound CO2ER hybrid catalyst with Bi/Bi2O3 nanoparticles (NPs) supported on the surface of nitrogen-doped reduced graphene oxide (NrGO) via a combined hydrothermal treatment with calcination process to partially reduce Bi2O3 into Bi. The Bi/Bi2O3 NPs with average diameter of ~20 nm was supported on NrGO nanosheets, which was denoted as Bi/Bi2O3/NrGO. Owing to the strong synergistic effect between Bi and Bi2O3, as-prepared Bi/Bi2O3/NrGO-700 hybrid catalyst displayed an impressive CO2ER activity and selectivity for formate production, featured by a Faradaic efficiency (F.E.) of 85%, a high electrolytic current density of -18 mA/cm2 with an applied potential of -0.9 V, a small onset potential of -0.5 V, as well as a low Tafel slope of 166 mV/dec. Additionally, the higher catalytic activity of Bi/Bi2O3/NrGO-700 for CO2ER than that of Bi/NrGO demonstrated that the bismuth-oxide-derived catalyst possessed a superior catalytic performance, which was attributed to the fact that partial reduction of Bi2O3 into Bi could increase the number of undercoordinated active Bi sites, thus resulting in increased CO2 adsorption and accelerated electron transfer processes, highly boosting CO2ER activity. We further combined the Bi/Bi2O3/NrGO-700 CO2ER catalyst with an oxygen evolution reaction (OER) catalyst of commercial Ir/C as a two-electrode system to realize highly active CO2ER and water splitting together when powered by planar silicon solar cell or two AA-size alkaline batteries.
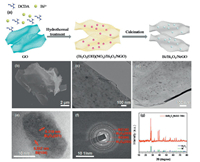
The fabrication of Bi/Bi2O3/NrGO hybrid was depicted in Fig. 1a. The mixture precursor was prepared by mixing GO solution, dicyandiamide (DCDA), and Bi(NO3)3·5H2O, and the obtained mixture was hydrothermally treated at 180 ℃ for 12 h, which resulted in the formation of the GO based precursor of (Bi2O2(OH)(NO3)/Bi2O3/NGO) composite (Fig. S1 in Supporting information) [11]. After further calcination at different temperatures in the range of 600-900 ℃, final product of Bi/Bi2O3/NrGO was obtained (details in Experimental section in Supporting information), hereafter abbreviated as Bi/Bi2O3/NrGO-x (x = 600, 700, 800 and 900).
|
Download:
|
| Fig. 1. (a) Schematic fabrication process of Bi/Bi2O3/NrGO. (b) FESEM image and (c-e) TEM and HRTEM images of Bi/Bi2O3/NrGO-700. (f) SAED pattern and (g) XRD pattern of Bi/Bi2O3/NrGO-700. | |
The morphology characterizations of as-prepared Bi/Bi2O3/ NrGO-700 hybrid were acquired by field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) images. The FESEM image (Fig. 1b) showed a nanosheet structure for Bi/Bi2O3/NrGO-700. The TEM images evidently proved the presence of large amounts of Bi/Bi2O3 NPs with diameter of ~20 nm, which were grown on the surface of NrGO nanosheets (Figs. 1c and d) [12]. Further high-resolution transmission electron microscopy (HRTEM) images clearly revealed that the Bi/Bi2O3 NPs were well confined into the NrGO nanosheets (Fig. 1e). The ordered lattice fringes with spaces of ~0.237 nm and ~0.319 nm, were associated with Bi (104) plane and Bi2O3 (201) plane, respectively. The corresponding selected area electron diffraction (SAED) pattern of Bi/Bi2O3/NrGO-700 showed four different diffraction rings and scattered dots, which could be attributed to crystal planes of Bi and Bi2O3 (Fig. 1f) [13].
The crystal structure of Bi/Bi2O3/NrGO-700 hybrid was identified by X-ray diffraction (XRD) and Raman spectroscopy. One broad peak located at 26.5° in the XRD pattern (Fig. 1g) was associated to the graphitic carbon, while the peaks at 27.2°, 37.9°, 39.6°, 44.6°, 48.7°, 62.2° and 64.5° were associated with the (012), (104), (110), (015), (202), (116) and (122) planes of metallic Bi (JCPDS No. 85-1329) [14], and the other distinct and sharp diffraction peaks at 27.9°, 31.8°, 32.7°, 46.2°, 46.9°, 54.3° and 55.5° were associated to the (201), (002), (220), (222), (400), (203) and (213) planes of Bi2O3 (JCPDS No. 27-0050) [15], respectively. Fig. S1 showed the comparison of XRD results recorded on the GO based composites after heating treatment at different temperatures of 600-900 ℃. Obviously, the crystalline structure of Bi/Bi2O3/NrGO-700 was similar with that of Bi/Bi2O3/NrGO-600 and Bi/Bi2O3/NrGO-800. Yet, the XRD pattern of Bi/Bi2O3/NrGO- 900 only exhibited one peak located at 26.5° corresponding to graphitic carbon. The Raman spectrum of Bi/Bi2O3/NrGO-700 was shown in Fig. 2a, in which two Raman bands centered at 71 and 98 cm-1 were associated with Eg and A1g templates of metallic Bi [13, 16], while the peaks of oxidation phases were found at the location of 313 and 410 cm-1. In the region above 500 cm-1, three clear peaks of D, G, and 2D bands were displayed at the locations of 1356, 1583, and 2689 cm-1, respectively. The ID/IG value of GO was determined to be 0.508 (Fig. S1), which was much smaller than that of Bi/Bi2O3/NrGO-700 with 0.891 (Fig. S2 in Supporting information), indicating the successful reduction of GO to rGO.

|
Download:
|
| Fig. 2. (a) Raman spectrum, (b) high resolution Bi 4f XPS spectrum, (b) high resolution O 1s XPS spectrum and (d) N2 adsorption desorption isothermal curve and the distribution of pore size (inset) of Bi/Bi2O3/NrGO-700. | |
X-ray photoelectron spectroscopy (XPS) of Bi/Bi2O3/NrGO-700 proved the presences of Bi, O, and N elements, and the doped N content was determined to be 1.57 at% (Fig. S3 in Supporting information). In high resolution Bi 4f XPS spectrum, four clear peaks at binding energies of 157.0, 159.4, 162.4, and 164.7 eV could be attributed to Bi 4f7/2, Bi3+ 4f7/2, Bi 4f5/2, and Bi3+ 4f5/2, respectively [17] (Fig. 2b). The high resolution O 1s XPS spectrum exhibited two peaks located at 531.8 and 530.3 eV ascribed to the Bi-O bonds, and one low-intensity peak located at 533.3 eV corresponding to hydroxide group (Fig. 2c) [18, 19]. Moreover, it could be seen that the content of Bi2O3 was decreased with the increase of calcination temperatures, which could be indicated by XPS spectrum of Bi 4f (Fig. S4 in Supporting information). It could be deduced that the fracture of Bi-O bonds at high temperature, led to the reduction and agglomeration of Bi2O3. In this process, part of the Bi2O3 was reduced to metallic Bi. The most optimal carbonization temperature was identified as 700 ℃ throughout this work. The content of Bi2O3 phase in Bi/ Bi2O3/NrGO-600 was much higher than that of in Bi/Bi2O3/NrGO- 700. However, the Bi/Bi2O3/NrGO-600 had a low degree of graphitization and poor conductivity, resulting in poor electron transfer ability. Thus, Bi/Bi2O3/NrGO-700 catalyst displayed a superior catalytic activity for CO2ER as compared to Bi/Bi2O3/NrGO-600. The N2 adsorption desorption isothermal curve of Bi/ Bi2O3/NrGO-700 displayed a Brunauer-Emmett-Teller (BET) surface area of 93 m2/g and pore size distribution diameter of 2.4 nm (Fig. 2d), which were supposed to enhance the exposed numbers of active sites [20]. Furthermore, thermogravimetric analysis (TGA) of Bi/Bi2O3/NrGO-700 revealed that the molar percentage of Bi achieved value of 17.6 at% (Fig. S5 in Supporting information).
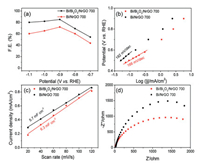
To investigate the electrocatalytic activity for CO2ER, the asprepared samples were evaluated in a CO2-saturated KHCO3 electrolyte (0.5 mol/L) within a conventional H-type threeelectrode cell. In this work, all potentials were converted versus reversible hydrogen electrode (RHE). The gaseous products of Bi/ Bi2O3/NrGO for CO2ER were measured by on-line gas chromatograph (GC), and liquid products were collected and analyzed by offline nuclear magnetic resonance (1H NMR) (Fig. S6 in Supporting information). The F.E. of formate production over as-prepared samples with different potentials was displayed in Fig. 3a. For both Bi/Bi2O3/NrGO-700 and Bi/NrGO catalysts, in suitable range of applied potentials, formate F.E. was improved with the potential applied more negatively and achieved the highest F.E. value of 85% and 72% at a potential of -0.9 V, respectively. However, formate F.E. decreased at more negative potentials than -0.9 V, which might be attributed to improvement of HER catalysis [21]. It was noteworthy that Bi/Bi2O3/NrGO-700 catalyst always gave the higher formate F.E. than control Bi/NrGO catalyst, which confirmed the excellent CO2ER activity of Bi/Bi2O3/NrGO-700. Fig. 3b showed Tafel slopes of Bi/Bi2O3/NrGO-700 and Bi/NrGO. It could be seen that the Tafel slope of 166 mV/dec for Bi/Bi2O3/NrGO-700 was lower than that of Bi/NrGO (182 mV/dec) under identical conditions, indicating that the reaction kinetics in Bi/Bi2O3/NrGO-700 catalyzed CO2ER process was faster than Bi/NrGO catalyzed one [8a, 22]. Moreover, electrochemical active surface areas (ECSA) of samples were investigated using electrochemical double layer capacitance (Cdl), that were tested with cyclic voltammetry (Fig. 3c and Fig. S7 in Supporting information). The Cdl values of Bi/Bi2O3/NrGO-700 and Bi/NrGO were calculated to be 6.3 and 5.7 m F/cm2, respectively. Since the ECSA was in direct proportional to the Cdl, one can deduce that the Bi/Bi2O3/NrGO-700 possessed a much larger ECSA than Bi/NrGO, which could help Bi/Bi2O3/NrGO-700 to expose more active sites during the CO2ER process [7, 9d]. Further, the smaller charge transfer impedance of Bi/Bi2O3/NrGO-700 than that of Bi/ NrGO was observed via electrochemical impedance spectroscopy (EIS), suggesting that the charge transfer ability of Bi/Bi2O3/NrGO- 700 was superior to that of Bi/NrGO (Fig. 3d). Obviously, Bi/Bi2O3/ NrGO-700 catalyst exhibited an excellent electrochemical performance relative to Bi/NrGO catalyst. On the basis of these results, one can conclude that the synergetic effect between Bi and Bi2O3 played a critical role in CO2ER [[8]].
|
Download:
|
| Fig. 3. (a) Formate F.E., (b) Tafel slopes, (c) Cdl values and (d) EIS Nyquist plots of Bi/ Bi2O3/NrGO-700 and Bi/NrGO-700. | |
To further investigate the influence of calcination temperatures on CO2ER performance, polarization curves of as-prepared Bi/Bi2O3/NrGO samples at different temperatures were measured in a Ar- or CO2- saturated KHCO3 electrolyte within a H-type three-electrode cell. As shown in Fig. 4a, onset potential of Bi/ Bi2O3/NrGO-700 was measured to be -0.7 V in Ar-saturated electrolyte, originated from its HER activity. After injecting CO2, onset potential of Bi/Bi2O3/NrGO-700 was increased to -0.5 V, along with the distinctly increased current density, demonstrating high CO2ER catalytic performance of Bi/Bi2O3/NrGO-700. The onset potential of -0.5 V from CO2ER catalyzed by Bi/Bi2O3/NrGO- 700 was lower than that of from the Bi/Bi2O3/NrGO-600 (-0.6 V), Bi/Bi2O3/NrGO-800 (-0.64 V), and Bi/Bi2O3/NrGO-900 (-0.68 V) (Fig. 4b), respectively, suggesting high CO2ER activity of Bi/Bi2O3/ NrGO-700. As shown in Fig. 4c, the generated formate was the main CO2ER product, together with minor amounts of H2 and CO gases. Obviously, formate F.E. for Bi/Bi2O3/NrGO-700 achieved the highest value of 85%, which was about 1.1, 1.6, and 2.7 times higher than that of Bi/Bi2O3/NrGO-600 (77%), Bi/Bi2O3/NrGO-800 (53%), and Bi/Bi2O3/NrGO-900 (32%), respectively, further confirming the superior CO2ER activity of Bi/Bi2O3/NrGO-700. Fig. 4d gave the comparison of recent representative CO2ER catalysts with Bi/Bi2O3/NrGO-700 for producing formate. Although partially reported CO2ER catalysts delivered a much higher formate F.E. than Bi/Bi2O3/NrGO-700, the applied potentials to achieve such high formate F.E. for these previously reported electrocatalysts were more negative than that for this Bi/Bi2O3/NrGO-700. To the best of our knowledge, such a high formate F.E. of 85% for Bi/ Bi2O3/NrGO-700 at a relatively positive potential of -0.9 V was superior to that of most of the previously reported CO2ER electrocatalysts for formate production (Table S1 in Supporting information). The superior formate selectivity of Bi/Bi2O3/NrGO- 700 was attributed to a combination of the synergistic effect between Bi and Bi2O3 and increased number of undercoordinated sites. As a contrast, the Bi/Bi2O3, Bi/NrGO, and Bi2O3/NrGO exhibited the certain activity for CO2ER (Fig. 4e). Nevertheless, the catalytic selectivity of these catalysts was far from satisfactory. The Bi/Bi2O3/NrGO-700 showed an obvious improved catalytic selectivity for formate production than the Bi/Bi2O3, Bi/NrGO, and Bi2O3/NrGO (Fig. 4f), proving the strong synergistic effect between Bi and Bi2O3.

|
(1) |

|
(2) |
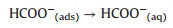
|
(3) |

|
Download:
|
| Fig. 4. (a) Polarization curves of Bi/Bi2O3/NrGO-700 in Ar and CO2-saturated 0.5 mol/L KHCO3. (b) Polarization curves in CO2-saturated 0.5 mol/L KHCO3 and (c) F.E. for CO, H2 and formate of Bi/Bi2O3/NrGO-600, Bi/Bi2O3/NrGO-700, Bi/Bi2O3/NrGO-800 and Bi/Bi2O3/NrGO-900. (d) Comparison of CO2ER performance for formate production from Bi/Bi2O3/NrGO-700 and other previously reported CO2ER electrocatalysts. (e) Formate F.E. of Bi/Bi2O3/NrGO-700, Bi/Bi2O3, Bi/NrGO and Bi2O3/NrGO. (f) F.E. for CO, H2, and formate of Bi/Bi2O3/NrGO with different contents of Bi(NO3)3·5H2O and DCDA. | |
It is noted that the CO2ER mechanism is usually dominated for supported Bi or Bi compounds catalysts [7, 9b]. Although specific reaction mechanism of Bi/Bi2O3/NrGO-700 involved in CO2ER to produce formate was still vague, the general reaction steps were illustrated as Eqs. (1–3). Firstly, dissolved CO2 molecules were captured by metal active sites to form the CO2(ads), then electron (e-) was transferred from the active sites to the CO2(ads) forming CO2·- intermediate. Next, the CO2·- intermediate was transformed into HCOO(ads)-intermediate throughone stepof cation coupledelectron transfer process on metal active sites. Finally, the HCOO(ads)- intermediate desorbed from metal active sites was transformed to form HCOO(aq)- ions dissolved in aqueous electrolyte.
Figs. 5a and b showed the partial current densities for formate and C1 (formate and CO) products of Bi/Bi2O3/NrGO samples, which reflected the CO2ER efficiency. The Bi/Bi2O3/NrGO-700 demonstrated a much higher partial currentdensityof -26.7 mA/cm2 than the Bi/Bi2O3/NrGO-600 (-19.5 mA/cm2), Bi/Bi2O3/NrGO-800 (-9.7 mA/cm2), and Bi/Bi2O3/NrGO-900 (-2.7 mA/cm2), respectively. The Bi/Bi2O3/NrGO-700 showed beneficial reaction kinetics with a Tafel slope of 166 mV/dec (Fig. 5c), which was lower than that of Bi/ Bi2O3/NrGO-600 (190 mV/dec), Bi/Bi2O3/NrGO-800 (201 mV/dec), and Bi/Bi2O3/NrGO-900 (209 mV/dec), indicating a beneficial kinetics of Bi/Bi2O3/NrGO-700 for the formation of CO2·- ntermediates [23]. Although the charge transfer impedance of Bi/Bi2O3/NrGO-800 and Bi/Bi2O3/NrGO-900 were smaller than that of Bi/Bi2O3/NrGO- 700 (Fig. 5d), the Bi/Bi2O3/NrGO-700 exhibited a superior catalytic activity for CO2ER with respect with Bi/Bi2O3/NrGO-800 and Bi/Bi2O3/NrGO-900, which could be attributed to the higher content of Bi2O3 in Bi/Bi2O3/NrGO-700. The ECSA of Bi/Bi2O3/NrGO was investigated according to the Cdl, which were tested with cyclic voltammetry (Fig. 5e) [8a]. The Cdl value of Bi/Bi2O3/NrGO-700 was 6.3 mF/cm2, higher than that of Bi/Bi2O3/NrGO-600 (5.3 mF/cm2), Bi/Bi2O3/NrGO-800 (4.7 mF/cm2), and Bi/Bi2O3/NrGO-900 (3.0 mF/cm2), indicating a remarkably large exposed numbers of active sites for Bi/Bi2O3/NrGO-700 [7, 9d]. Additionally, Bi/Bi2O3/ NrGO-700 maintained a stable current density of -18 mA/cm2 at -0.9 V over 10 h associated with the average formate F.E. of 85%, suggesting a long-term stability of Bi/Bi2O3/NrGO-700 for CO2ER (Fig. 5f).

|
Download:
|
| Fig. 5. (a) Partial current densities for formate production, (b) partial current densities for C1 production, (c) Tafel slopes, (d) EIS Nyquist plots and (e) Cdl of Bi/Bi2O3/NrGO-600, Bi/Bi2O3/NrGO-700, Bi/Bi2O3/NrGO-800 and Bi/Bi2O3/NrGO-900. (f) Stability and formate F.E. of Bi/Bi2O3/NrGO-700 at -0.9 V. | |
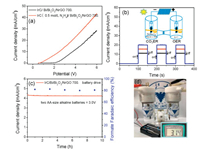
Encouraged by the excellent CO2ER performance of Bi/Bi2O3/ NrGO-700, we further pursued a two-electrode device that employed Bi/Bi2O3/NrGO-700 supported on carbon paper as cathode for CO2ER and commercial Ir/C supported on carbon paper as anode for OER to explore practical application. As shown in Fig. 6a, polarization curve of CO2ER-OER couple was measured to achieve a potential of ~3.77 V at 10 mA/cm2 in 0.5 mol/L KHCO3 electrolyte, and after adding N2H4, it displayed superior activity with a potential of ~2.51 V at 10 mA/cm2. In addition, polarization curve of the two-electrode CO2ER-OER couple showed an onset potential of ~1.9 V. After adding N2H4, the onset potential was decreased to ~0.6 V, suggesting more effective energy conversion efficiency of this couple system achieved with the adding of N2H4. Notably, overall catalytic performance of Bi/Bi2O3/NrGO-700-Ir/C was superior to that of Bi-Ir/C that was employed for CO2ER-OER device (onset potential of ~2.1 V) [7]. Moreover, Bi/Bi2O3/NrGO- 700 was further combined with Ir/C to achieve CO2ER-OER electrolysis powered by a plane silicon photovoltaic cell at impressive energy conversion efficiency. The CO2ER-OER device showed a fast and repeatable dynamic photocurrent response following continuous on/off illumination circulation under simulated sunlight illumination (AM 1.5 G, 100 mW/cm2), indicating an excellent charge transmission capacity of this CO2ER-OER device (Fig. 6b). Furthermore, we used two AA-size alkaline batteries (potential = 3.0 V) to drive the designed system for CO2ER-OER electrolysis (Figs. 6c and d). The current density of batteries-driven CO2ER-OER cell had a subtle variation after continues reaction over 2 h, and then tended to be stable. Notably, the CO2ER-OER device could maintain a stable current density of ~ 4.3 mA/cm2 over 10 h along with an average formate F.E. of 81.2%, suggesting practical feasibility of using two AA-size alkaline batteries to drive CO2ER-OER device.
|
Download:
|
| Fig. 6. (a) Polarization curves of two-electrode CO2ER-OER cell in 0.5 mol/L KHCO3 with and without 0.5 mol/L N2H4. (b) Photocurrent densities of CO2ER-OER device in 0.5 mol/L KHCO3 at different applied potentials of 3.5, 4.0 and 4.5 V powered by solar panel under shredded simulated sunlight. (c) Chronoamperometric measurement and formate F.E. of batteries-driven CO2ER-OER device. (d) Digital picture of CO2ER-OER device driven by two AA-size alkaline batteries. | |
In summary, we developed a hybrid electrocatalyst composed of Bi/Bi2O3 NPs with an average diameter of ~20 nm supported on NrGO for CO2ER. The Bi/Bi2O3/NrGO-700 catalyst displayed an impressive CO2ER catalytic activity for formate production to achieve the highest formate F.E. of 85% at -0.9 V with a high current density of -18 mA/cm2, a small onset potential of -0.5 V and a low Tafel slope of 166 mV/dec, which was competitive with the other reported Bi-based CO2ER catalysts. The excellent catalytic activity and selectivity of Bi/Bi2O3/NrGO-700 could be attributed to the increased numbers of undercoordinated Bi active sites that originated from the reduction of Bi2O3, which accelerated the intermediate reaction steps during the CO2ER catalysis. Additionally, the Bi/Bi2O3/NrGO-700 was combined with commercial Ir/C as a two-electrode system for efficient CO2ER-OER electrolysis which was powered by planar silicon solar cell or two AA-size alkaline batteries. The Bi/Bi2O3 supported on NrGO hybrid designed in this work can pave a promising pathway for synthesizing other metal/metal oxide derived catalysts to enhance their CO2ER catalytic activity and raise the possibilities that metal oxides may be involved in CO2ER pathways on other metal catalyst.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsF. He thanks the support of the Natural Science Foundation of Zhejiang Province (No. LR16E080003). Y. Hou thanks the support of National Natural Science Foundation of China (Nos. 21922811, 51702284, 21878270), Zhejiang Provincial Natural Science Foundation of China (No. LR19B060002), the Fundamental Research Funds for the Central Universities, and the Startup Foundation for Hundred-Talent Program of Zhejiang University.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.031.
| [1] |
(a) F. Li, L. Chen, G.P. Knowles, D.R. MacFarlane, J. Zhang, Angew. Chem. Int. Ed. 56 (2017) 505-509; (b) C. Lu, J. Yang, S. Wei, et al., Adv. Funct. Mater. 29 (2019) 1806884; (c) T. Wang, Q. Zhao, Y. Fu, et al., Small Methods 3 (2019) 1900210; (d) W. Xiong, J. Yang, L. Shuai, et al., ChemElectroChem 6 (2019) 5951-5957; (e) W. Zheng, J. Yang, H. Chen, et al., Adv. Funct. Mater. 30 (2019) 1907658; (f) W. Zheng, C. Guo, J. Yang, et al., Carbon 150 (2019) 52-59; (g) C. Wang, Y. Zhao, H. Xu, et al., Appl. Catal. B 263 (2020) 118314; (h) X. Wu, C. Wang, Y. Wei, et al., J. Catal. 377 (2019) 309-321; (i) Y. Zhao, Y. Wei, X. Wu, et al., Appl. Catal. B 226 (2018) 360-372. |
| [2] |
L. Sun, V. Reddu, A.C. Fisher, X. Wang, Energy. Environ. Sci. 13 (2020) 374-403. DOI:10.1039/C9EE03660A |
| [3] |
E. Irtem, T. Andreu, A. Parra, et al., J. Mater. Chem. A 4 (2016) 13582-13588. DOI:10.1039/C6TA04432H |
| [4] |
Z.B. Hoffman, T.S. Gray, K.B. Moraveck, T.B. Gunnoe, G. Zangari, ACS Catal. 7 (2017) 5381-5390. DOI:10.1021/acscatal.7b01161 |
| [5] |
J. Schneider, H. Jia, K. Kobiro, et al., Energy. Environ. Sci. 5 (2012) 9502-9510. DOI:10.1039/c2ee22528j |
| [6] |
C. Yan, H. Li, Y. Ye, et al., Energy. Environ. Sci. 11 (2018) 1204-1210. DOI:10.1039/C8EE00133B |
| [7] |
N. Han, Y. Wang, H. Yang, et al., Nat. Commun. 9 (2018) 1320. DOI:10.1038/s41467-018-03712-z |
| [8] |
(a) S. Gao, Y. Lin, X. Jiao, et al., Nature 529 (2016) 68-71; (b) H. Mistry, A.S. Varela, C.S. Bonifacio, et al., Nat. Commun. 7 (2016) 12123; (c) Y. Chen, M.W. Kanan, J. Am. Chem. Soc. 134 (2012) 1986-1989. |
| [9] |
(a) S. Liu, X.F. Lu, J. Xiao, X. Wang, X.W.D. Lou, Angew. Chem. Int. Ed. 58 (2019) 13828-13833; (b) Q. Gong, P. Ding, M. Xu, et al., Nat. Commun. 10 (2019) 13828-13833; (c) Y. Zhang, F. Li, X. Zhang, et al., J. Mater. Chem. A 6 (2018) 4714-4720; (d) C.C. Miao, G.Q. Yuan, ChemElectroChem 5 (2018) 3741-3747. |
| [10] |
(a) H. Zhong, Y. Qiu, T. Zhang, et al., J. Mater. Chem. A 4 (2016) 13746-13753; (b) J.H. Koh, D.H. Won, T. Eom, et al., ACS Catal. 7 (2017) 5071-5077. |
| [11] |
H. Huang, Y. He, X. Li, et al., J. Mater. Chem. A 3 (2015) 24547-24556. DOI:10.1039/C5TA07655B |
| [12] |
C. Liu, X. Huang, J. Liu, et al., Adv. Sci. 7 (2020) 1901480. DOI:10.1002/advs.201901480 |
| [13] |
W. Zhang, Y. Hu, L. Ma, et al., Nano Energy 53 (2018) 808-816. DOI:10.1016/j.nanoen.2018.09.053 |
| [14] |
M. Ahila, M. Malligavathy, E. Subramanian, D.P. Padiyan, Solid State Ionics 298 (2016) 23-34. DOI:10.1016/j.ssi.2016.10.017 |
| [15] |
J. Hou, C. Yang, Z. Wang, et al., Appl. Catal. B 142- 143 (2013) 504-511. |
| [16] |
Y. Wang, J. Zhao, Y. Zhu, et al., Colloids Surf. A 434 (2013) 296-302. DOI:10.1016/j.colsurfa.2013.05.078 |
| [17] |
Y. Yu, C. Cao, H. Liu, et al., J. Mater. Chem. A 2 (2014) 1677-1681. DOI:10.1039/C3TA14494A |
| [18] |
P.T. Babar, A.C. Lokhande, B.S. Pawar, et al., Appl. Surf. Sci. 427 (2018) 253-259. |
| [19] |
G.H. Jiang, X. Li, Z. Wei, et al., Acta Metall. Sin. 28 (2015) 460-466. DOI:10.1007/s40195-015-0220-1 |
| [20] |
W. Tian, H. Zhang, H. Sun, et al., Adv. Funct. Mater. 26 (2016) 8651-8661. DOI:10.1002/adfm.201603937 |
| [21] |
Q. Lai, N. Yang, G. Yuan, Electrochem. Commun. 83 (2017) 24-27. DOI:10.1016/j.elecom.2017.08.015 |
| [22] |
C.W. Li, J. Ciston, M.W. Kanan, Nature 508 (2014) 504-507. DOI:10.1038/nature13249 |
| [23] |
X. Li, W. Bi, M. Chen, et al., J. Am. Chem. Soc. 139 (2017) 14889-14892. DOI:10.1021/jacs.7b09074 |
 2020, Vol. 31
2020, Vol. 31 

