b Department of Radiology, Xiang'an Hospital of Xiamen University, Xiamen 361102, China;
c Department of Radiological Intervention, The First Hospital of China Medical University, Shenyang 110001, China
Primary liver cancer is one of the most common malignant tumors of the digestive system [1]. Hepatocellular carcinoma (HCC) occupies the vast majority of liver cancer, becoming the fourth leading cause of cancer death worldwide in recent years [2]. The curative treatment strategy for early stage HCC patients includes resection, transplantation and ablation [3]. Currently, due to the poor diagnosis in clinical practice, only 10%–15% of HCC patients are suitable for resection and transplantation [4]. Transcatheter hepatic arterial chemoembolization (TACE) is the most popular palliative treatment, which is an intervention for those patients with advanced liver cancer who are not suitable for surgery. By injecting an embolization agent that contains chemotherapeutics into the liver tumor area via a microcatheter under the guidance of digital subtraction angiography (DSA) through arterial vessels, TACE is believed to suppress the growth of tumors [5].
TACE prevents the growth of cancer by blocking blood supply to the tumor, leading to ischemic necrosis while simultaneously injecting chemotherapeutics in situ for further chemotherapy [6]. Since intrahepatic tumors are supplied by the hepatic artery and hepatic tissue is mainly supplied by the hepatic portal vein, artery occlusion would lead to necrosis of tumor cells. Due to its minimal trauma and wide indications, TACE can greatly ameliorate the quality of life and survival time of patients with HCC in clinical practice [7, 8]. However, incomplete embolization, poor controllable release of drugs, deterioration of hypoxia, and other factors will affect the degree of tumor necrosis after TACE, leading to poor therapeutic effect with high postoperative tumor residual [4]. In particular, the deterioration of hypoxia, the improvement of chemotherapy tolerance of tumor cells [9], the increase in angiogenesis [10], and the aggravation of immunosuppression [11] induced by TACE will promote tumor recurrence and metastasis.
In the past decades, the application of nanotechnology in the field of medicine has received widespread attention. More importantly, nanotechnology with different structures and properties have been developed that show great application prospects in the imaging diagnosis and treatment of cancer [12, 13]. For example, the coordination-driven self-assembly of metal-organic nanoformulations have realized the combined application of fluorescence/photoacoustic imagingguided photothermal therapy and gene therapy for cancer [14]. Such multifunctional hybrid nanoparticles provide a "win-win" strategy for cancer treatment. The application of gene-edited cell membrane vesicles as a multifunctional targeted delivery platform, with its good biocompatibility and convenience of biological function modification, is widely exploited in the immunotherapy and combination therapy with chemotherapy, photothermal therapy and other therapies of various cancer models [15-18]. In addition, the tumor microenvironment-corresponding nanostructures obtained based on nanotechnology, significantly improved the infiltration depth of therapeutic drugs to solid tumors, inducing favorable effects for tumor inhibition [19]. These multifunctional nanosystems in the early diagnosis and detection of cancer [20, 21], targeted delivery of drugs, regulation of tumor microenvironment, stimulation of immune system, and combination therapy in preclinical studies have provided a rich repository of strategies for the clinical treatment of cancer [22-24]. It is believed that the currently unsatisfactory efficacy and limitation of application of TACE in the treatment of HCC will be ameliorated by its combination with nanotechnology [25, 26].
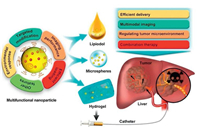
Recently, the blooming intersection of TACE and nanomedicine has received wide attention and achieved remarkable progressin the treatment of HCC with multiple therapeutic approaches [27]. In addition, the reasonable introduction of imaging probes with advanced nanocompositesgive the visualization of embolization and multimodal monitoring for efficient drug delivery [28, 29]. In this review, based on theamelioration of TCAE application via nanomaterials, it is briefly summarized in accordance with efficient drug delivery, nanotheranostic-based real-time monitoring and combination treatment, which are the most critical aspects that affect the clinical application and efficacy of TACE (Fig. 1). Moreover, the exploration directions and prospects in the future personalized medicine such as hypoxia improvement and immune enhancement in TACE were discussed and summarized, so as to provide some ideas for follow-up studies.
|
Download:
|
| Fig. 1. Scheme of TACE for liver cancer using embolization agents in combination with multifunctional nanomaterials. | |
2. Efficient drug delivery
Clinically, chemotherapeutic drugs are usually mixed directly with the embolization agent (e. g., lipiodol) to form an emulsion for TACE treatment. Although this method is straightforward and practical, the loaded drug is volatile in this state, and will be quickly eluted from the embolization agent following injection. The rapid release can lead to high concentrations of drugs into the circulatory system and induce acute side effects. Additionally, even local administration in the tumor region cannot solve the problems of non-specificity, high toxicity, and rapid clearance of chemotherapeutic drugs [30].
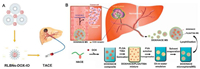
Fortunately, nanocarriers with characteristics of adequate drug loading and intelligent release will markedly improve the effect of chemotherapy for cancer treatment. Recently, drug-loaded nanoparticles dispersed in an embolization agent, iodized oil, and embolization microspheres have been commonly used for TACE treatment. Recently, Quan et al. designed stable reversed lipid-based nanoparticles (RLBNs) in lipiodol for carrying the hydrophilic doxorubicin (DOX) (Fig. 2A) [27]. RLBNs differ from a simple mixture of drug and oil solution in that it is a homogeneous system with hydrophobic nanostructures that disperse significantly in oil. The prepared RLBNs load DOX between the juxtaposing polar core and the highly biocompatible lipid materials with stable nanostructure. Interestingly, in this stable state, DOX will be released continuously, which extends the drug's retention time. When compared to conventional TACE treatment, RLBNs loaded with DOX in lipiodol were more effective at suppressing the growth of tumors in Wistar rats.
|
Download:
|
| Fig. 2. (A) Scheme of RLBNs in lipiodol designed for carrying hydrophilic chemotherapeutics for TACE. Reproduced with permission [27]. Copyright 2019, American Chemical Society. (B) Scheme of emulsification method for TACE with PLGA microspheres incorporating DOX and HACE in a spatiotemporal manner. Reproduced with permission [33]. | |
Drug-eluting beads (DEBs), known as drug-carrying embolization microspheres, are also commonly used as embolization carrier agents in clinical TACE, in which the release of therapeutic drugs can be controlled during treatment [31]. However, DEB-based TACE treatments nevertheless still present some shortcomings, such as ineffective treatment, incomplete tumor response, and adverse reactions. Thus, the ideal properties of drug-carrying embolic materials, such as targeted delivery, imaging performance, tumor permeability, biodegradability, biocompatibility, and ability to achieve multiple therapeutic modalities must be leveraged. Taking on the advantages of nanocarriers, the multifunctional "nano-on-micro" delivery systems were newly developed. Lee et al. synthesized nanopar-ticle-containing gellan gum (GG) embolic microspheres through the water-in-oil emulsification method as the vehicle for DOX [32]. The hyaluronic acidcontained in short-chain hyaluronic acid/ polyethylenimine/doxorubicin (sHH/PH/DOX) nanoparticles can increase the affinity of nanoparticulate systems with cancer cells through the CD44 receptor. Besides, the grafted polyethylenimine (PEI) features high transfection capability, which can facilitate the transport of medicine into HepG2 liver cancer cells and boost the IC50 value of the HepG2 cell once merged into the GG micro-spheres. Furthermore, GG/sHH/PH/DOX microspheres exhibited a good embolization effect in rabbit ear arteries.
It is believed that placing functionalized nanoparticles in embolic microspheres and elevating targeted delivery of drugs could significantly elevate the efficacy of TACE. Cho et al. developed poly(lactic-co-glycolic acid) (PLGA) microspheres incorporating DOX and hyaluronic acid-ceramide (HACE) in a spatiotemporal manner, which modified the emulsification method for TACE (Fig. 2B) [33]. DOX and HACE could release from PLGA microspheres following self-assembly into nano-particles after microspheres were biodegraded in artificial biological fluids. The DOX-HACE nanoparticles further enhanced the cellular internalization efficiency of DOX in HepG2 and McA-RH7777 liver cancer cells. Notably, the strategy of improved penetration depth and delivery targeting induced excellent suppression of tumor growth in McA-RH7777 tumor-implanted rat models after intra-arterial (IA) administration. Furthermore, it has been reported that antagonizing integrin expression in the tumor environment can prevent tumor recurrence and metastasis [34]. To achieve targeted delivery of integrin inhibitors, Vogl et al. applied superparamagnetic iron oxide (SPIO) nanoparticles to load GRGDSP, which is a Gly-Arg-Gly-Asp-Ser-Pro integrin inhibitor including an RGD peptide to further combine with TACE [35]. In their study, the application of GRGDSP-loaded nanoparticles in TACE significantly inhibited tumor growth via the superiority of nanoparticle delivery relative to the control group, but their therapeutic mechanisms and imaging advantages have not been well studied.
3. Nanotheranostic-based real-time monitoringIodide oil is radiopaque and the injection of iodide oil containing chemotherapy drugs can be observed in real time under DSA. Moreover, the deposition and embolization of iodide oil in the tumor area can be evaluated by computed tomography (CT) detection after IA administration. However, evidence indicates scarcely any significant correlation between the iodine content in the tumor area and the chemotherapy level of the tumor. In other words, the CT detection of iodized oil could not be used as a reliable indicator of local drug concentration [36, 37].
In addition to their ability to load drugs, nanoparticles with imaging properties can provide richer imaging information for treatment. For example, SPIOs have been widely used as magnetic resonance imaging (MRI) contrast agents and drug delivery carriers [38, 39]. Recently, porous magnetic nano-clusters (pMNCs) with porous structures and carboxylic groups developed by Kim et al. afford high-drug-loading efficiency, unremitting drug release, and high MRI T2-weighted image contrast [40]. DOX-pMNCs within iodinated oil provides a stronger signal for MRI monitoring while improving the efficacy of liver cancer after infusion via IA administration to target rabbit VX2 orthotopic liver cancer.
At present, applied DEBs are not visible by X-ray or MRI, leading to the delivery and embolization status of DEBs, which cannot be directly monitored. The newly developed multifunctional "nano-on-micro" delivery system with a contrast agent, embolization agent, and chemotherapy agent can be used as an ideal carrier for selective arterial catheter-guided TACE [31]. The visible microspheres in multi-mode MRI or CT can directly display the delivery of these crafts containing medicine. Thus, it appears that novel multifunctional nano-on-micro formulations can overcome the shortcomings of traditional TACE and are expected to be the most effective non-surgical treatment for HCC [25].
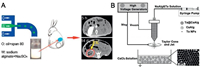
Barium alginate (ALG) microspheres containing BaSO4 nano-particles (BaSO4/ALG microspheres) in situ were synthesized via a one-step microfluidic technique according to Shen et al. (Fig. 3A) [41]. Evenly dispersing in the form of ALG microspheres, the existence of radiation-opaque BaSO4 nanoparticles were capable of directly detecting the embolization efficiency and the position of microspheres in the TACE process under X-ray, which offers great promise for direct and real-time monitoring. For the same purpose, hoping to endow embolism microspheres with radiopaque properties, Yang et al. designed calcium alginate microspheres that load tantalum nanoparticles (Ta@CaAlg) via a one-step electrospray (Fig. 3B) [28]. The intrinsically radiopaque microspheres that were prepared yielded high drug loads and controllable drug release. Ta@CaAlg microspheres, in particular, demonstrated excellent X-ray imaging and embolization in normal renal arteries under digital radiography and CT. As expected, those preponderances of Ta@CaAlg microspheres ameliorated the accuracy of embolization according to the location and distribution of embolization agents during TACE surgery through real-time feedback under DSA.
|
Download:
|
| Fig. 3. (A) Schematic illustration of BaSO4/ALg microspheres in situ synthesized via a one-step microfluidic technique. Reproduced with permission [41]. Copyright 2015, American Chemical Society. (B) Schematic illustration of Ta@CaAlg via a one-step electrospray. Reproduced with permission [28]. Copyright 2018, Ivy spring International Publisher. | |
MRI-capable embolic microspheres have received widespread attention given that they can be used for noninvasive post-operative monitoring while concurrently reducing radiation exposure. Lee et al. designed a therapeutic magnetic microcarrier (TMMC) which is a chitosan microsphere containing SPIOs with capability of MRI detection for anti-cancer embolotherapy [42]. Deformable chitosan with SPIOs (SPIO-chitosan MS) was prepared by crosslinking the ionic sensitized gel and genipin with polyethylene glycol (PEG) as a pore-forming agent. In their work, magnetic imaging performance of SPIO-chitosan MS with different concentrations of SPIO was studied in vivo and in vitro, and the subsequent embolization was identified in the renal artery of rabbit by MRI at 18 weeks. Analogously, poly(acrylic acid) microspheres (PMs) that proved to be an ideal embolic agent were initially prepared by Fan et al. using inverse suspension polymerization [43]. The morphology, elasticity and magnetic property of SPIO-loaded PMs (SPMs) were studied to evaluate embolization and MRI detectability in vitro and in vivo.
Additionally, magnetic nanoparticles can be used not only as imaging agents but also for efficient drug delivery. Accordingly, MRI-visible amonafide (AMN)-eluting alginate microspheres were synthesized using a highly efficient microfluidic gelation process by Larson et al. for targeted arterial-infusion chemotherapy [44]. The microspheres included magnetic clusters formed by SPIOs possessing biocompatibility, MRI contrast, and sustained release of AMN. Afterwards, operability of AMN-loaded magnetic microsphere-applied TACE infusions and visualization of the intra-hepatic delivery in MRI was immediately demonstrated in a rat hepatoma model. Recently, the feasibility of TMMCs, such as magnetic iron-cobalt nanoparticle-encapsulated degradable PLGA particles developed by Martel et al. was verified in a channel mimicking the rabbit liver artery [45]. TMMCs are coated with multiple layers of graphite shells to reduce the saturation magnetization (Ms) reduction in the packaging step exhibiting high Ms required for the MRI steering and the advisable mean diameter for arterial embolism. In a later in vivo study [46], TMMCs were used for directional steering to the target blood vessel under the intervention of magnetic resonance navigation (MRN). Meanwhile, tracking accumulation of particle and the assessment of the efficiency of MRN can be achieved via a hypointense signal induced by TMMC. This kind of multifunctional embolism microspheres markedly facilitates the selection of drug delivery, visualization of in vivo distribution of embolization microspheres, persistence of drug release, and penetration depth of the drug in the tumor for hepatoma therapy [47].
4. Combination treatmentBased on the advanced drug delivery nanosystems, the combination of several treatments (i. e., chemotherapy, radiotherapy, surgery, or TACE) can greatly improve the effect of cancer therapy. For example, hydrogels have excellent biocompatibility and water absorption expansion properties, which are widely used in the field of biomedicine and have been used as embolization agents in TACE of HCC. With the hydrogel as a carrier and a sequence of the multifunctional nanometer system, Liu et al. achieved controlled release of drugs in a mouse subcutaneous tumor model [48], and used photodynamic therapy [49], chemotherapy [50], local radiotherapy [51], surgical resection [52], and other treatment methods combined with immunotherapy to eradicate the tumor and significantly inhibit tumor recurrence and metastasis.
If combined with TACE, this treatment strategy may yield better results in the treatment of HCC. Li et al. designed hollow gold nanospheres (HAuNS) as nanocarriers that could be used for drug loading, drug tracking, and photothermal ablation (PTA) of the tumor [29]. PEG-modified HAuNS (PEG-HAuNS) can be marked with radioactive elements 64Cu for detecting biological distribution of nanocarriers and verifying the loading of potent vascular disrupting agents such as combretastatin A-4 phosphate disodium (CA4P). Treatment with ethiodized oil-mingling CA4P combined with DOX@PEG-HAuNS for TACE can not only facilitate the effective conveyance of chemotherapy drugs and thereby optimize the effect of chemotherapy, but also trigger the ablation effect of local photothermal therapy on tumors under near-infrared irradiation, reducing the damage to normal tissues. Granov et al. developed a silicone composite material with magnetic nanopar-ticle that can be used for arterial embolization combined with hyperthermia [53]. The silicone elastomer matrix composite material doped with magnetic nanoparticles has been proved to exhibit excellent efficiency in thermal conversion and thermal expansion under alternating magnetic fields in vitro. Through the addition of potassium iodide, it can be endowed with opaque properties for CT imaging. This compound, which can generate heat and deforms upon magnetic interference, can achieve local high-heat ablation on cancer cells while amplifying the degree of vascular closure for the suppression of tumors.
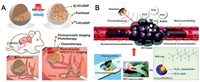
Recently, Song et al. encapsulated hollow copper nanoparticles (HCuSNPs) loading paclitaxel (PTX) in PLGA microspheres and further labeled them with radioactive iodine-131 (i. e., 131I-HCuSNPs-MS-PTX as a clinical integration complex) (Fig. 4A) [54]. The integrator was capable of combining with local photothermal therapy and radiotherapy to destruct the tumor after interventional arterial administration. It is worth mentioning that 131I-HCuSNPs-MS-PTX containing HCuSNPs with photoacoustic imaging performance and 131I with single-photon emission computed tomography (SPECT) imaging performance provides multiple imaging detection tools for this treatment strategy. Su et al. also developed mPEG-PLGA@ZrO2@(ILs+ DOX) microspheres with PEG modification of PLGA microcapsule-implanting DOX carried hollow ZrO2 nanoparticles and microwave (MW)-sensitive ionic liquid insurance-linked securities (ILS) for combining MW ablation with TACE in a rabbit liver cancer model [55]. More sophisticated imaging techniques need to be developed to monitor therapeutic responses. Li et al. blended perfluorohexane (PFH), Fe3O4 and PLGA integrated multifunctional Fe3O4-PFH/PLGA nanocapsules into lipiodol via interventional injection into the tumor for synergizing high-intensity focused ultrasound (HIFU) ablation and TACE (Fig. 4B) [56]. The ultrasound, MRI and photoacoustic tri-modality imaging systems were successfully applied for treatment guidance and monitoring, showing great potential for future clinical translation.
|
Download:
|
| Fig. 4. (A) Schema shows HCuSNPs loading PTX in PLGA microspheres and further labeled them with radioactive iodine-131 (i. e., 131I-HCuSNPs-MS-PTX as a clinical integration complex). Reproduced with permission [54]. Copyright 2018, Ivyspring International Publisher. (B) Schematic illustration of multifunctional nanocapsules into lipiodol via interventional injection into the tumor for synergizing HIFU ablation and TACE. Reproduced with permission [56]. Copyright 2016, Royal Society of Chemistry. | |
5. Problems and perspective
After TACE, the tumor environment tends to deteriorate due to the interruption of oxygen and blood supply induced by vascular obstruction. Aggravated hypoxia after embolization is the driving factor of vascular endothelial growth factor (VEGF) expression [10]. VEGF as a biomarker of tumor angiogenesis is directly involved in tumor growth and metastasis of liver cancer [57]. Relevant studies have suggested that hypoxia is an important factor in promoting angiogenesis [58] and tumor growth [59]. If there is existence of incomplete embolization during TACE, postoperative hypoxia of tumor tissue will expedite the growth of tumors. To solve this problem, oxygen supply needs to be supplied during TACE. Crosslinked tetramer hemoglobin has been used as an artificial oxygen donor for tumors after TACE [60]. It was found that apoptosis and necrosis of tumor cells were promoted due to delivery of cisplatin-and hemoglobinbased oxygen carriers in situ rat HCC models. Furthermore, multifunctional nanovectors can provide oxygen for the tumor microenvironment via acid-catalyzed oxygen release or hydrogen peroxide reaction in the tumor area. In early research, by regulating hypoxia, the tolerance of cancer cells to drugs can be reduced [61] and the sensitivity to radiotherapeutic [62], photodynamic [63], and sonodynamic therapy [64] can be enhanced [65]. The application of this multifunctional nanocarrier in TACE of HCC may bring new opportunities to mediate the progression of HCC [26].
The tumor microenvironment is an immunosuppressive environment that provides favorable conditions for the immunological escape of cancer cells. In recent years, cancer immunotherapy has received unprecedented attention [66]. Moreover, research on nanosystem-based tumor immunotherapy has made extensive progress, yielding remarkable results in the treatment of cancers when combined with traditional treatment strategies such as surgery [52], chemotherapy [67], photothermal therapy [68], and radiotherapy [69] among others. On the other hand, embolization leads to tumor tissue necrosis and secretion of tumor-related antigens, which may induce a systemic immune response; however, this effect is too weak to effectively inhibit local recurrence and distant metastasis. Embolization also reduces the number of peripheral T helper cells, thereby weakening the antitumor immune response [70]. At the same time, the hypoxic microenvironment caused by embolization leads to up-regulation of HIF-1α and PD-L1 expression in the tumor, amplifying the immunosuppressive effect [71]. Therefore, in the process of TACE treatment, multifunctional nanosystems can regulate the immune system by, for example, suppressing immune checkpoints or activating immune cells, which will have great potential for ameliorating the therapeutic effect of HCC.
Despite its reaches, there are certain conjectural limiting factors for the application of nanotechnology in TACE that depend predominantly on multidisciplinary cooperation between clinical intervention doctors, chemists/physicists/materials scientists, and imaging specialists. The regulatory hurdles to nanomaterials inTACE, such as safety and efficacy, remain roadblocks for clinical translation. Additionally, TACE operation must be conducted under the guidance of DSA, and postoperative efficacy monitoring by CT and MRI. These devices are usually available altogether only in hospitals, but not in general laboratories. Therefore, if researchers cooperate with clinicians for combining technology, facilities, and research literacy, treatment efficacy may dramatically improve. With continuous efforts by multidisciplinary approaches, the use of nanotechnology in TACE will shed new light on the treatment of HCC.
6. ConclusionsIn summary, as a conservative treatment for patients who cannot undergo surgical treatment, TACE can improve treatment efficacy and survival time to some extent. However, it requires improvements in its unsatisfactory efficacy due to uncontrollable drug delivery, insufficient imaging monitoring, hypoxic aggravation, neovascularization, and immunosuppression, among other problems. The application of nanotechnology in the medical field brings new light to the diagnosis and treatment of tumors. Through continuous exploration by researchers, nanomaterials have achieved remarkable results in the field of HCC for clinical translation. Combined with the previous research, the application of nanotechnology in TACE has opened a new door for liver cancer therapy. Although some work on nanomaterials combined with TACE has seen preliminary exploration in animals, it is mainly focused on enhancing controllable delivery of drugs and enhancing imaging methods, which are relatively basic. More strikingly, based on nanotechnology, the combined application of thermal ablation, MW ablation, radiotherapy, and other treatment methods with TACE has achieved remarkable results in animal models, and the endowed multi-modal imaging of detection methods has greatly enriched the diagnosis and treatment strategies of HCC. However, the exploration of hypoxia, neovascularization, and immunosup-pression caused by TACE would accelerate the development of effective medical products by exploiting nanotechnology, and it is believed to become a new avenue of exploration to improve TACE treatments.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Major State Basic Research Development Program of China (No. 2017YFA0205201), the National Natural Science Foundation of China (Nos. 81925019, 81422023 and U1705281), the Fundamental Research Funds for the Central Universities (Nos. 20720190088 and 20720200019), and the Program for New Century Excellent Talents in University, China (No. NCET-13-0502).
| [1] |
Y. A. Ghouri, I. Mian, J. Rowe, J. Carcinog. 16 (2017) 1. |
| [2] |
T. Vos, A. A. Abajobir, K. H. Abate, et al., Lancet 390 (2017) 1211-1259. DOI:10.1016/S0140-6736(17)32154-2 |
| [3] |
A. Forner, M. Gilabert, J. Bruix, J. L. Raoul, Nat. Rev. Clin. Oncol. 11 (2014) 525-535. DOI:10.1038/nrclinonc.2014.122 |
| [4] |
A. Marcos-Alvarez, R. L. Jenkins, W. K. Washburn, et al., Arch. Surg. 131 (1996) 292-298. DOI:10.1001/archsurg.1996.01430150070014 |
| [5] |
R. Lencioni, Semin. Oncol. 39 (2012) 503-509. DOI:10.1053/j.seminoncol.2012.05.004 |
| [6] |
M. Tsurusaki, T. Murakami, Liver Cancer 4 (2015) 165-175. DOI:10.1159/000367739 |
| [7] |
J. M. Llovet, M. Ducreux, R. Lencioni, et al., J. Hepatol. 56 (2012) 908-943. DOI:10.1016/j.jhep.2011.12.001 |
| [8] |
P. Viveiros, A. Riaz, R. J. Lewandowski, et al., Cancers 11 (2019) 1085. DOI:10.3390/cancers11081085 |
| [9] |
J. P. Lai, A. Conley, B. S. Knudsen, et al., Histopathology 67 (2015) 442-450. DOI:10.1111/his.12623 |
| [10] |
K. Liu, X. L. Min, J. Peng, et al., J. Clin. Med. Res. 8 (2016) 297-302. DOI:10.14740/jocmr2496w |
| [11] |
X. Chang, X. Lu, J. Guo, et al., Cancer Treat. Rev. 74 (2019) 49-60. DOI:10.1016/j.ctrv.2018.08.006 |
| [12] |
W. Fan, B. Yung, P. Huang, et al., Chem. Rev. 117 (2017) 13566-13638. DOI:10.1021/acs.chemrev.7b00258 |
| [13] |
Y. Shi, T. Lammers, Acc Chem, Res. 52 (2019) 1543-1554. DOI:10.1021/acs.accounts.9b00148 |
| [14] |
C. Chu, E. Ren, Y. Zhang, et al., Angew. Chem. Int. Ed. 58 (2019) 269-272. DOI:10.1002/anie.201812482 |
| [15] |
G. Lin, Y. Zhang, L. Zhang, et al., Nano Res. 13 (2020) 238-245. DOI:10.1007/s12274-019-2606-2 |
| [16] |
P. Lv, X. Liu, X. Chen, et al., Nano Lett. 19 (2019) 2993-3001. DOI:10.1021/acs.nanolett.9b00145 |
| [17] |
P. Zhang, L. Zhang, Z. Qin, et al., Adv. Mater. 30 (2018) 1705350. DOI:10.1002/adma.201705350 |
| [18] |
X. Zhang, Y. Zhang, Y. Zhang, et al., Biomater. Sci. 8 (2020) 1575-1579. DOI:10.1039/C9BM02088H |
| [19] |
P. Zhang, J. Wang, H. Chen, et al., J. Am. Chem. Soc. 140 (2018) 14980-14989. DOI:10.1021/jacs.8b09396 |
| [20] |
Q. Lin, Z. Li, Q. Yuan, Chin. Chem. Lett. 30 (2019) 1547-1556. DOI:10.1016/j.cclet.2019.06.016 |
| [21] |
C. Yan, L. Shi, Z. Guo, et al., Chin. Chem. Lett. 30 (2019) 1849-1855. DOI:10.1016/j.cclet.2019.08.038 |
| [22] |
R. Tong, D. S. Kohane, Annu. Rev. Pharmacol. Toxicol. 56 (2016) 41-57. DOI:10.1146/annurev-pharmtox-010715-103456 |
| [23] |
J. He, C. Li, L. Ding, et al., Adv. Mater. 31 (2019) 1902409. DOI:10.1002/adma.201902409 |
| [24] |
X. Wang, J. Sheng, M. Yang, Chin. Chem. Lett. 30 (2019) 533-540. DOI:10.1016/j.cclet.2018.10.010 |
| [25] |
D. H. Kim, A. C. Larson, Interv. Oncol. 360 (2016) E173-E182. |
| [26] |
L. Xiao, M. Wang, Cell Biochem. Biophys. 70 (2014) 269-272. DOI:10.1007/s12013-014-9893-8 |
| [27] |
L. Shen, Y. Zhang, J. Zhang, et al., ACS Appl. Mater. Interfaces 11 (2019) 20642-20648. DOI:10.1021/acsami.9b03110 |
| [28] |
J. Zeng, L. Li, H. Zhang, et al., Theranostics 8 (2018) 4591-4600. DOI:10.7150/thno.27379 |
| [29] |
J. Li, M. Zhou, F. Liu, et al., Radiology 281 (2016) 427-435. DOI:10.1148/radiol.2016152510 |
| [30] |
F. Deschamps, T. Isoardo, S. Denis, et al., Acta Biomater. 87 (2019) 177-186. DOI:10.1016/j.actbio.2019.01.054 |
| [31] |
D. Bannerman, W. Wan, Expert Opin. Drug Deliv. 13 (2016) 1289-1300. DOI:10.1080/17425247.2016.1192122 |
| [32] |
M. F. Hsu, Y. S. Tyan, Y. C. Chien, et al., Sci. Rep. 8 (2018) 731. DOI:10.1038/s41598-018-19191-7 |
| [33] |
S. Y. Lee, J. W. Choi, J. Y. Lee, et al., Drug Deliv. 25 (2018) 1472-1483. DOI:10.1080/10717544.2018.1480673 |
| [34] |
C. Chen, R. Tian, Y. Zeng, et al., Bioconjug. Chem. 31 (2020) 276-292. DOI:10.1021/acs.bioconjchem.9b00734 |
| [35] |
J. Qian, E. Oppermann, A. Tran, et al., World J. Gastroenterol. 22 (2016) 5042-5049. DOI:10.3748/wjg.v22.i21.5042 |
| [36] |
R. T. Poon, H. Ngan, C. M. Lo, et al., J. Surg. Oncol. 73 (2000) 109-114. DOI:10.1002/(SICI)1096-9098(200002)73:2<109::AID-JSO10>3.0.CO;2-J |
| [37] |
A. Parvinian, L. C. Casadaban, Z. Z. Hauck, et al., Diagn. Interv. Radiol. 21 (2015) 235-240. DOI:10.5152/dir.2014.14394 |
| [38] |
W. Cai, Y. Zhang, J. Wang, et al., Chem. Eng. J. 380 (2020) 122473. DOI:10.1016/j.cej.2019.122473 |
| [39] |
Y. Shi, J. Wang, J. Liu, et al., Biomaterials 233 (2020) 119753. DOI:10.1016/j.biomaterials.2019.119753 |
| [40] |
M. J. Jeon, A. C. Gordon, A. C. Larson, et al., Biomaterials 88 (2016) 25-33. DOI:10.1016/j.biomaterials.2016.02.021 |
| [41] |
Q. Wang, K. Qian, S. Liu, et al., Biomacromolecules 16 (2015) 1240-1246. DOI:10.1021/acs.biomac.5b00027 |
| [42] |
E. Y. Chung, H. M. Kim, G. H. Lee, et al., Carbohydr. Polym. 90 (2012) 1725-1731. DOI:10.1016/j.carbpol.2012.07.058 |
| [43] |
H. Wang, X. Y. Qin, Z. Y. Li, et al., Int. J. Pharm. 511 (2016) 831-839. DOI:10.1016/j.ijpharm.2016.07.028 |
| [44] |
D. H. Kim, J. Chen, R. A. Omary, et al., Theranostics 5 (2015) 477-488. DOI:10.7150/thno.10823 |
| [45] |
P. Pouponneau, J. C. Leroux, S. Martel, Biomaterials 30 (2009) 6327-6332. DOI:10.1016/j.biomaterials.2009.08.005 |
| [46] |
P. Pouponneau, J. C. Leroux, G. Soulez, et al., Biomaterials 32 (2011) 3481-3486. DOI:10.1016/j.biomaterials.2010.12.059 |
| [47] |
P. Pouponneau, G. Soulez, G. Beaudoin, et al., Cardiovasc. Intervent. Radiol. 37 (2014) 784-790. DOI:10.1007/s00270-013-0770-4 |
| [48] |
H. Ruan, Q. Hu, D. Wen, et al., Adv. Mater. 31 (2019) 1806957. DOI:10.1002/adma.201806957 |
| [49] |
Z. Meng, X. Zhou, J. Xu, et al., Adv. Mater. 31 (2019) 1900927. |
| [50] |
S. Yu, C. Wang, J. Yu, et al., Adv. Mater. 30 (2018) 1801527. DOI:10.1002/adma.201801527 |
| [51] |
Y. Chao, L. Xu, C. Liang, et al., Nat. Biomed. Eng. 2 (2018) 611-621. DOI:10.1038/s41551-018-0262-6 |
| [52] |
Q. Chen, C. Wang, X. Zhang, et al., Nat. Nanotechnol. 14 (2019) 89-97. |
| [53] |
I. S. Smolkova, N. E. Kazantseva, K. N. Makoveckaya, et al., Mater. Sci. Eng. C 48 (2015) 632-641. DOI:10.1016/j.msec.2014.12.046 |
| [54] |
Q. Liu, Y. Qian, P. Li, et al., Theranostics 8 (2018) 785-799. DOI:10.7150/thno.21491 |
| [55] |
J. Mao, S. Tang, D. Hong, et al., Nanoscale 9 (2017) 3429-3439. DOI:10.1039/C6NR09862B |
| [56] |
Y. You, Z. Wang, H. Ran, et al., Nanoscale 8 (2016) 4324-4339. DOI:10.1039/C5NR08292G |
| [57] |
G. Niu, X. Chen, Curr. Drug Target. 11 (2010) 1000-1017. DOI:10.2174/138945010791591395 |
| [58] |
J. M. Llovet, M. I. Real, X. Montaña, et al., Lancet 359 (2002) 1734-1739. DOI:10.1016/S0140-6736(02)08649-X |
| [59] |
C. M. Lo, H. Ngan, W. K. Tso, et al., Hepatology 35 (2002) 1164-1171. DOI:10.1053/jhep.2002.33156 |
| [60] |
X. B. Liu, Q. Cheng, W. Geng, et al., Artif. Cells Nanomed. Biotechnol. 42 (2014) 229-236. DOI:10.3109/21691401.2013.808647 |
| [61] |
H. Chen, F. Li, Y. Yao, et al., Theranostics 9 (2019) 90-103. DOI:10.7150/thno.30259 |
| [62] |
J. Li, W. Shang, Y. Li, et al., Int. J. Nanomedicine 13 (2018) 5925-5936. DOI:10.2147/IJN.S173914 |
| [63] |
G. Yang, J. Tian, C. Chen, et al., Chem. Sci. 10 (2019) 5766-5772. DOI:10.1039/C9SC00985J |
| [64] |
X. Qian, Y. Zheng, Y. Chen, et al., Adv. mater. 28 (2016) 8097-8129. DOI:10.1002/adma.201602012 |
| [65] |
Y. Wang, W. Shang, M. Niu, et al., Int. J. Nanomedicine 14 (2019) 3705-3722. DOI:10.2147/IJN.S196959 |
| [66] |
M. Goldberg, Nat. Rev. Cancer 19 (2019) 587-602. DOI:10.1038/s41568-019-0186-9 |
| [67] |
Q. Song, Y. Yin, L. Shang, et al., Nano Lett. 17 (2017) 6366-6375. DOI:10.1021/acs.nanolett.7b03186 |
| [68] |
Q. Chen, L. Xu, C. Liang, et al., Nat. Commun. 7 (2016) 13193. DOI:10.1038/ncomms13193 |
| [69] |
K. Lu, C. He, N. Guo, et al., Nat. Biomed. Eng. 2 (2018) 600-610. DOI:10.1038/s41551-018-0203-4 |
| [70] |
H. Takaki, N. Imai, T. T. Contessa, et al., J. Vasc. Interv. Radiol. 27 (2016) 1561-1568. DOI:10.1016/j.jvir.2016.01.150 |
| [71] |
M. Z. Noman, G. Desantis, B. Janji, et al., J. Exp. Med. 211 (2014) 781-790. DOI:10.1084/jem.20131916 |
 2020, Vol. 31
2020, Vol. 31 





