b College of Materials Science and Engineering, Sichuan University, Chengdu 610064, China;
c Institute of Cytology of the Russian Academy of Sciences(RAS), St. Petersburg 194064, Russia
Nanoparticles have been widely used in anti-tumor therapy, while the therapeutic efficacy is not that satisfying. One of the most important reasons is that nanoparticles with small sizes cannot accumulate in tumor sites and would be pumped back in bloodstream, while those large-sized nanoparticles can accumulate in tumor sites but cannot penetrate deeply and kill the innermost tumor cells [1-3]. The dilemma makes it difficult to balance both retention and penetration of nanoparticles at the same time.
As one of the most important physicochemical properties of nanoparticles, size plays an important role not only in tumor sites but influence nanoparticles'behavior from beginning to end. Mononuclear phagocytic system (MPS), consists of blood monocytes, dendritic cells and tissue-resident macrophages [4], is the first barrier that nanoparticles encounter after being intravenous injected into body. MPS internalizes foreign nanodrugs by phagocytosis, and the formed internal phagosome is the destination of most foreign nanodrugs to be degraded, which accordingly leads to insufficient accumulation in tumor sites. Fang et al. reported that there was a dramatic reduction of uptake by MPS with sizes reducing [5]. Nevertheless, this does not mean that the smaller, the better. Nanoparticles below 50 nm and 5.5 nm are easily sequestered by liver and kidney respectively, and will be rapidly cleared up [6, 7]. Then nanoparticles with sizes range from 200 nm to 1.2 μm could get through the pore of vessels and get into tumor sites. It is reported that after entering into tumors, large nanoparticles around 150 nm are the advisable choice for retention [8-11], and small nanoparticles (~10 nm) show talents in deep penetration [12, 13]. Numerous researches focus on finding nanoparticles'ideal size with superior distributive activity. However, finding a balance between penetration and retention seems to be a negotiation that large-sized nanoparticles cannot penetrate deeply into tumors while small-sized nanoparticles is easily pumped back into bloodstream exhibiting poor tumor retention. The original design of nanoparticles with fixed sizes always needs to compromise and sacrifice both penetration and retention efficacy, which affect treatment efficiency. Recent days, intelligent aggregatable nanoparticles provided a solution to the distressing dilemma [14]. The original size of nanoparticles is usually small enough to get rid of MPS and enter in deep tumor; once triggered by specific stimulus, nanoparticles tend to aggregate and prolong the retention time in tumor. Those kinds of nanoparticles consist of special materials or functionalized with responsive groups, and specifically undergo transformation through hydrogen bond, π-π stacking and hydrophobic interactions, etc. Nanoparticles after transformation showed a relatively large size, which are also called nanoaggregates.
In the review, we focus on the recent advances in design of nanoaggregates. There are many stimuli that can be utilized, such as internal (enzyme and pH) and external stimuli (light, temperature and reactive oxygen species (ROS)), which will be introduced in detail. Then, we summarize combination of aggregation strategy with tumor microenvironment modulation. The synergy treatment makes the nanoaggregates better exert anti-tumor efficacy. In the end, the opportunities and challenges of nanoaggregates are discussed for further direction.
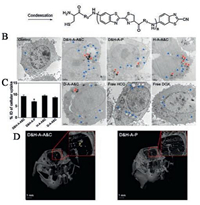
2. Enzyme-induced aggregations 2.1. Enzyme-induced click reactionsIn the development of tumor, it often exhibits some specific characteristics different from other normal tissues, and numerous studies have demonstrated that the direct cause of these abnormal activities is the dysregulation of enzymes [15]. There are many enzymes that are reported to be highly expressed in tumor sites, such as legumain, matrix metalloproteinase (MMP), hyaluronidase (HAase) and furin. And because of the highly selectivity and biofriendly reactions catalyzed by enzymes, the enzymeinduced aggregation is commonly used in designing nanoparticles compared with other stimuli. Among which, click reactions stand out for the high reaction efficiency and the simplest reaction method. The mostly used chemical groups for click reactions are sulfhydryl and maleimide [16], azide and terminal alkynes [17], 1,2-thiolamino and cyano [18], etc. Liang et al. did great works in click reactions between 1,2-thiolamino and cyano groups, and provided potential applications under different stimuli (Fig. 1A) [19]. By decorating different substrates on the thiol and amino group, the condensation reaction could be precisely controlled through pH change, disulfide reduction or proteolytic hydrolysis [20-22]. Among which, the two proteases (furin and caspase-3) and their corresponding cleavage substrates (Arg-X-Lys/Arg-Arg↓X and Asp-Glu-Val-Asp↓X, X can be any residue and ↓ indicates the cleavage site) were mostly studied [23]. Furthermore, our group utilized the same click reaction with the substrate of legumain (Ala-Ala-Asn↓Cys-Lys), which is overexpressed in glioma sites [24-26]. Briefly, we designed two parts of gold nanoparticles (GNPs) modified with different chemical groups:the legumain responsive peptide Ala-Ala-Asn-Cys-Lys (AK) and 2-cyano-6-aminobenzothiazole (CABT) respectively. The two parts of gold GNPs only aggregated in glioma sites once AK was cleaved by legumain, and the aggregation was obvious after 24 h (Figs. 1B-D). The GNPs-A & C strategy demonstrated very successful anti-tumor result, and the median survival time increased by 288% comparing with saline groups. In addition, the aggregated GNPs showed an increased absorption in near-infrared region between 650 nm and 900 nm, endowing with an extraordinary multispectral optoacoustic tomography (MSOT) imaging, which could provide a more precise position of glioma site comparing with other fluorescence imaging. The enzyme-induced aggregation has been utilized widely including noninvasive imagination of protein activity [27, 28], positron emission computed tomography (PET) [29-31], overcoming multidrug resistance [32], and magnetic resonance imaging MRI [33].
|
Download:
|
| Fig. 1. (A) Schemes of a controlled click reaction based on 2-cyanobenzothiazole (CBT) and 1,2-aminothiol. Copied with permission [19]. Copyright 2014, Royal Society of Chemistry. (B) TEM images of C6 cells after incubation with D & H-A-A & C and control formulations for 24 h, red arrows represent AuNPs aggregates, blue arrows represent autophagic vacuoles. (C) Percentage incubated dose (% ID) of cellular uptake of D & H-A-A & C and control nanoparticles after incubation with C6 cells for 24 h. (D) Three-dimensional volume-rendering images of C6 gliomabearing mice brains after intravenous injection with D & H-A-A & C and D & H-A-P for 24 h, red dash line represents the mock gold morphology using the collected signal at glioma site. Reproduced with permission [24]. Copyright 2019, American Chemical Society. | |
Azide and alkyne group was another active catalyst-free click reaction [34]. Wang et al. introduced a recognition-reactionaggregation (RRA) cascade strategy to form an in situ peptidebased superstructure on the renal cell membrane with the assistance of click reaction between azide and alkyne (Fig. 2A) [35]. The carbonic anhydrase IX (CAIX)-targeted peptide was modified on dibenzocyclooctyne (P1-DBCO) to ensure the precise combination with renal cancer cell membrane, click reaction happened after P2-N3 was consequently introduced and the synthesized P3 simultaneously aggregated into superstructures owing to the extended hydrophobic unit and unbalanced hydrophilic-hydrophobic interactions. The peptide-based aggregation on the renal cancer cell membrane perturbed the membrane permeability and enhanced the influx of chemo-drugs, sensitizing drug toxicity with IC50 that significantly reduced by nearly 3.5-fold comparing with free DOX.

|
Download:
|
| Fig. 2. (A) Schematic illustration of the molecular structure and the RRA cascade process. Copied with permission [35]. Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) TEM images of the nanostructures formed by 1-OMe-OP (100 μmol/L) before and after the addition of ALP or both ALP and CES. In PBS (pH 7.4);scale bar=100 nm. (C) Viabilities of HepG2 and OVSAHO cells treated with 1-OMe-OP. Reproduced with permission [40]. Copyright 2017, American Chemical Society. | |
2.2. Enzyme-induced self-assembly
In addition to aggregations through click reactions, the properties of nanoparticles themselves can also be used. There are some nanoparticles can self-assembly into different shape or size after stimulation, and the majority of them are composed of amphiphilic block copolymers. If properly designed, the nanoparticles will undergo destruction, formation or morphological transformation through amphiphiles changes or electrostatic interactions [36]. Alkaline phosphatases (ALPs) are widely used to construct self-assembly of nanofibers in vivo, as the hydrophilic and hydrophobic properties of block copolymers always have a change after phosphorylation and dephosphorylation [37]. Xu et al. designed the D-tetrapeptide with the N-terminal capped with a naphthyl group. The phosphotyrosine residue on D-tetrapeptide was dephosphorylated and turned into self-assembly molecules to form nanofibers with naphthyl group through π-π stacking. Similarly, Liang et al. utilized the ALPs to induce hierarchical selfassemblies, while the naphthyl group was replaced by CABT motif which was also a good π-π stacking supplier (Cys (SEt)-Glu-Tyr (H2PO3)-Phe-Phe-Gly-CBT) [38]. The aggregation happened in two cascade steps. The first-order ALP-instructed self-assembly happened in extracellular environment because of the specific expression site of ALPs, which yielded the amphiphilic fragment of nanofibers (Cys (SEt)-Glu-Tyr-Phe-Phe-Gly-CBT). And the second-order self-assembly further happened after entering into cells where the disulfide bond was reduced by GSH to trigger the click reaction between 1,2-thiolamino and Cys, the same click reaction which has been mentioned in the earlier section. By employing a click reaction during the synthesis of nanofibers, the postsynthesized nanofibers appeared to have enhanced mechanical strength, providing a potential in post-modulation of supramolecular nanofibers. Moreover, ALPs in combination with other enzymes could achieve selective aggregation and avoid unspecific accumulation in major organs [39]. Feng et al. first utilized the down-regulated enzyme in cancer cells to reduce systemic toxicity caused by accumulation in non-target sites [40]. Briefly, they designed peptidic precursors as the substrates of both carboxylesterases (CES) and ALPs. The dephosphorylation by ALP turned the precursors into self-assembly molecules to form nanofibers, while CES cleavage resulted in disassembly of the formed nanofibers (Fig. 2B). The reversed assembly and disassembly could selectively generate accumulation in cancer cells expressing ALP and downregulating CES (e. g., OVSAHO) while being innocuous to cells that up-regulate CES (e. g., HepG2). Cell viability experiment further suggested that IC50 of OVSAHO cells was 22 μmol/L, while it was almost harmless to HepG2 cells (Fig. 2C). Though this strategy has not been used in vivo, it is of great potential for metastatic cancers, such as ovarian cancers into liver, and can also be used to eliminate the damage to normal tissues.
3. pH-Triggered aggregationBecauseof the hypoxia microenvironment and the unique aerobic glycolysis process, tumor sites exhibit mildly acidic microenvironment at around pH 6.5, and the pH goes even more lower in endosomes and lysosomes which is about 5.0~5.5, while the blood and normal tissuesmaintain a relatively neutralpH at around 7.2~7.4 [41]. Quantities of research studies decorated pH-sensitive surface molecules on nanoparticles to ensure an instantaneous aggregation. The commonly used pH-responsive molecules are zwitterionic compounds, such as cytochrome c [42], 2,3-dimethylmaleic anhydride (DMMA) [43], 2-dimethylaminoethyl methacrylate (DMAEMA) [44] and hydrolysis-susceptible amides. These molecules keep stable at neutral condition, but once into the acidic tumor microenvironment, the broken charge balance leads to nanoaggregates. Moreover, some peptides and macromolecules can also be used to form pHsensitive precursor due to their special compositions with both acid and base. Ferguson et al. reported a family of oligopeptides which were flanked by Asp-terminated tetrapeptide wings that displayed pH-triggered assembly into supramolecular aggregates [45], and the oligopeptide repeat domain of a melanosomal protein was also reported pH-sensitive [46]. Proteins with isoelectric point around physiological pH of tumor can be ideal pH-responsive nanocarriers such as hemoglobin [47]. The pH-responsive aggregations are achieved through electrostatic interactions, which happen very quickly once the charge balance is broken. Therefore, the ultrasensitivityof pH-responsive nanoparticles comparedwith enzymeinduced aggregation, assure a much lower risk of nanoparticles being pumped back to bloodstream before the aggregation happen. Jutaek et al. designed a pH-responsive smart GNPs [48], which were 10 nm in size and decorated with the hydrolysis-susceptible citraconic amide units. GNPs maintained negative charge during the circulation of blood stream, but once into the mildly acidic tumor microenvironment, the citraconic amide units changed to positive charge, while the other unhydrolyzed parts still showed negative charge, leading to immediate aggregation through electrostatic interactions between the two opposite charged groups, with the diameter changed from 14 nm to 200 nm. To better respond to multiple pH in different parts in vivo, Ni et al. designed a selfassemble molybdenum (Mo)-based polyoxometalate (POM) clusters [49], and the size of POM increased in a cascade with pH decreased from 7.4 to 6.5 and to 5.5(Fig. 3). The initial sizes of the clusters are ultrasmall with only 1.9 nm, which can escape from recognition and capture by liver and spleen. Once entering low pH environment of 6.5, these clusters were found to self-assemble to around 25 nm in diameter, and would further aggregate to 0.43 μm at pH 5.0 in cells through hydrogen bond after protonation of POM. The pH-responsive POM made full use of pH differences with pH 6.5-6.8 in extracellular tumor milieu and pH 5.0-6.0 in endocytic organelles, which enhanced the tumor retention of POM over time.

|
Download:
|
| Fig. 3. (A) TEM images of clusters at pH 7.4, 6.5 and 5.0. (B) DLS measurements of Ox-POM clusters with successive acidifications from pH 7.4 to 6.5 and to 5.0. Reproduced with permission [49]. Copyright 2017, American Chemical Society. | |
4. Light-induced aggregation
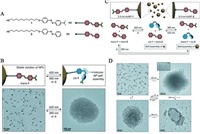
External stimuli such as light has been broadly used in stimulitriggered transformation of nanoparticles. The use of external stimuli can prevent the cytotoxicity to normal tissues and make the transformation process more controllable. Compared to internal stimuli such as pH and enzyme, light irradiation possesses the advantage of spatial and temporal controllability. In drug delivery system, the premature drug release at undesirable site is a key problem of low delivery efficacy. While the problem can be solved by adjusting the irradiation artificially [50]. The morphology change only happens where the light is cast, and the loaded drug inside light-responsive nanoparticles is released afterwards, which is a good way to guarantee an on-demand drug release. More importantly, lots of new materials have been developed which specifically respond to irradiation with longer wavelength, giving solutions to the weak tissue permeability of shorter wavelength light [51]. Azobenzenes [52], diazirine and spirobenzopyran are commonly used photoactive groups [53, 54], and structure changes will happen through C-C, C-H, O-H, and X-H insertions after UV-vis irradiation. Interestingly, Manna et al. reported that the required wavelength can be adjusted according to the chemical groups modified on azobenzene. They first introduced NP-1, which could self-assemble under irradiation at 420 nm and disassemble with a subsequent irradiation at 365 nm (Figs. 4A and B). Then they introduced NP-2 which exhibited isomerization under irradiation at 365 nm while returned back after irradiated with 420 nm (Figs. 4C and D) [55]. By placing both types of azobenzenes on the same nanoparticles, they created unique materials whose selfassembly is induced irrespective of the wavelength of incident light. Cheng et al. decorated GNPs with diazirine groups, and the aggregation happened very soon from 20 nm to 346 nm in only 25 min after irradiation. The aggregated GNPs showed a very promising character in photothermal effect, when irradiated with an 808 nm laser, the temperature of the aqueous solution raised to 57.1 ℃, which can be further used in photothermal therapy and photoacoustic imaging in vivo [56].
|
Download:
|
| Fig. 4. (A) Structural formulas of the thiolated azobenzenes used in this study. (B) Reversible isomerization of 1 on the surface of a nanoparticle (upper) and TEM images of 1-functionalized Au NPs before (left) and after (right) exposure to blue (420 nm) light. Aggregates such as the one on the right could be disassembled using UV (365 nm) light or by gentle heating (down). (C) Components of the system:1-functionalized 2.5 nm Au NPs and 2-functionalized 5.5 nm Au NPs (upper) and mechanism of self-assembly and disassembly after irradiation or heat (down). (D) TEM images of NPs after changes in the UV-vis spectra. Reproduced with permission [55]. Copyright 2015, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | |
5. ROS-induced aggregation
Recent days, ROS-responsive materials have been developed in drug delivery system because of the high level of ROS in tumor sites. However, most of them are used in prodrug delivery with the specifically ROS-responsive thioketal cleavage [57, 58], while few works were reported in design of nanoaggregates. Lee et al. first reported that the solubility of bilirubin was changed after exposure to ROS [59]. Our group fully utilized this property and designed a linear chimeric triblock molecule with controllable transformation ability (Fig. 5) [60]. Phe-Phe-Val-Leu-Lys (FFVLK) peptide functioned as hydrogen bonds to form nanofibers; bilirubin and PEG functioned as hydrophobic head and hydrophilic tail respectively. The formed micelles were stable and soluble in blood circulation, but instantaneously transformed into nanofibers once the solubility of bilirubin was changed by ROS in tumors. The ROS-responsive nanofibers extremely prolonged retention in tumor sites and exhibited superior suppression of tumor with inhibition rate of 61.8%. Even though the ROS level in tumor sites is reported higher than normal tissues, the currently used ROS-responsive molecules still needs an extra trigger to induce more sufficient ROS for quick response, and the mostly used strategy is co-delivery with photosensitive molecules such as chlorin e6 and pheophorbide a [61, 62]. In addition to provide stimulation, ROS is also a widely known toxic reagent, which can do damage to tumor cells [63]. The combination therapy could not only improve the response sensitivity but also kill tumor cells by photodynamic therapy, which is also called a "one stone, two birds" strategy.

|
Download:
|
| Fig. 5. (A) Structures of the linear chimeric triblock molecules and schematic illustration of the construction and the transformation of PTX2-TK@Ce6/BR-FFVLK-PEG. The enhanced intratumoral retention resulted from the transformation and the simultaneous combinational therapy are also shown in the scheme. (B) TEM images of PTX2-TK@Ce6/BR-FFVLK-PEG with different Ce6/BR ratios and their changes post 650 nm irradiation. Reproduced with permission [60]. Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | |
6. Temperature-triggered aggregation
Temperature-sensitive polymers could undergo phase transitions and form nanoaggregates after the temperature change. There are two main mechanisms:upper critical solution temperature (UCST) and lower critical solution temperature (LCST) [64, 65]. Aggregations will happen whether the temperature above the LCST or below the UCST, and more importantly, the responded temperature can be regulated through polymer modification [66], providing a more flexible and diverse choices for the design of nanoparticles. The most direct way to construct a temperatureresponsive nanoplatform is to modify the thermo-sensitive polymers on the outlayer of nanoparticles and inject intratumorally (Fig. 6A), giving a temperature change from 25 ℃ to 37 ℃ [67]. Araki et al. chose to give an external heating around the tumor sites after intravenous injection of nanoparticles (Fig. 6B), so that the local temperature of tumor was higher than LCST and ensure the accuracy of aggregation site [68]. However, both the two strategies are not that suitable and smart enough for application in vivo, and nanoparticles with changeable LCST are deemed to have more potential in the future development. When applied in vivo, the LCST of the initial polymers are required to be greater than 37 ℃ to ensure the successful penetration into tumor with relatively small sizes. While after entering into the target sites and treated with corresponding specific stimulation, the LCST is then expected to decrease below 37 ℃, leading to the aggregation of polymers. The change of LCST usually needs assistance with another stimulus such as pH or enzyme, and as the responded temperature is not that easy to control, the temperature-triggered aggregations tend to be more complicated and require more accuracy at the beginning of design.
|
Download:
|
| Fig. 6. (A) Scheme of the thermally triggered in vitro and in vivo assembly of ELP-AuNPs for X-ray computed tomographic (CT), photothermal (PT), photoacoustic (PA) imaging and photothermal therapy (PTT) of tumor. Copied with permission [67]. Copyright 2017, American Chemical Society. (B) Schematic illustration of differences in morphology and temperature between normal and tumor tissues. Tumor tissue exhibits void spaces corresponding to the EPR effect. Copied with permission [68]. Copyright 2017, American Chemical Society. | |
Poly (N-substituted acrylamide)s are the most frequently studied temperature-sensitive polymers with LCST, including poly (N, N'-diethylacrylamide) [69], poly (2-carboxyisopropylacrylamide) [70]. Matsumoto et al. designed a pH-and temperatureresponsive protein nanoparticles [71], which was composed of poly (N-isopropylacrylamide-co-acrylic acid)(pNIPAAm-co-AA). The polymer had an LCST above 60 ℃, allowing for a stable circulation in blood. When entering acidic tumor sites, AA monomer units were protonated and the changed hydrophobic interactions dominated to a decreased LCST, which was tested to be 44 ℃ at pH 6 and 31.9 ℃ at pH 5 respectively, leading to phase transition-caused aggregation. This temperature sensitive strategy in combination with enzyme-and pH-specific response can not only prolong nanocarriers retention but also can be applied in realtime therapy evaluation, which has never been reported before and has a great prospect.
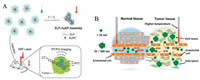
7. Synergy of tumor microenvironment modulating and aggregationThough great successes have been made in aggregation strategies as mentioned before, there is still much more room for improvement in anti-tumor effect as the efficient delivery is hindered due to the heterogeneity of tumor microenvironment. The abnormal leaky vascular system, elevated interstitial fluid pressure, and the dense ECM of tumor microenvironment lead to restricted extravasation and penetration of nanoparticles, which in turn affect the efficient delivery. To deliver nanoparticles to tumor sites as much as possible, researchers developed various ways to modulate tumor microenvironment such as vascular endothelial growth factor (VEGF) receptor-2 blocking antibody for vascular normalization, collagenase for ECM degradation [72]. Cediranib is an anti-angiogenesis drug, which normalizes the vascular abnormalities and emerges as an efficient complementary treatment in anti-tumor combination therapy [73]. To increase the delivery of nanoparticles to tumor sites and reduce the unexpected backflow to vessels, our group combined the aggregation strategy with tumor vessel normalization [74]. By treating cediranib two days before intravenous injection of GNPAK and GNP-CABT, the retention of aggregated GNP-A & C was significantly enhanced. Li et al. co-delivered cetuximab together with nanoparticles by directly modified on CuS NPs [75]. The modification of cetuximab impressively inhibited the formation and progression of tumor vessels, and enhanced the aggregation of CuS NPs in tumor.
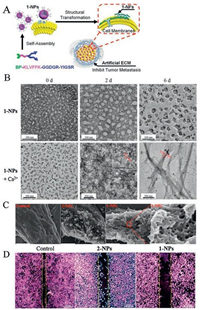
Except for the additional treatment of drugs to modulate the tumor microenvironment, the nature of aggregated nanoparticles can also be used in microenvironment modulation. Degradation of ECM by enzyme is considered the main source of metastasis, and researchers tried every possible way to inhibit the procedure. Hu et al. got inspiration from natural process of extracellular matrix (ECM) deposition and designed transformable nanomaterials for inhibiting tumor invasion and metastasis [76]. They mimicked the natural self-assembly way of fibrous proteins into organized mesh, which was induced by mechanical forces from in-cell actin filament through the binding of protein-ligands, to construct an in situ transformable nanomaterials (BP-KLVFFK-GGDGR-YIGSR)(Fig. 7A). The as-designed nanomaterials first accumulated in tumors after transforming into nanofibers with the existence of Ca2+, and further entwined to form network around solid tumors (Figs. 7B and C), which was thought to be an artificial ECM (AECM) to avoid tumor invasion (Fig. 7D). The AECM existed in tumor site over 72 h and exhibited extraordinary anti-metastasis effect with the inhibition rates of 82.3% and 50.0% in breast and melanoma tumor models respectively. Membrane protein targeted peptide could also be introduced to construct superstructure on membrane as described in session 2.1. The adhesion of superstructure perturbed the membrane effectively and enhanced the influx of chemo-drugs, which solved the problem of tumor cell drug resistance. Same work was reported by Wang et al. to form nanoaggregates on the membrane of cancer-associated fibroblasts [77].
|
Download:
|
| Fig. 7. (A) Schematic illustration of the biomimic construction of AECM based on transformable 1-NPs for high-efficient inhibition of tumor invasion and metastasis. (B) TEM images for the corresponding morphologies of nanoaggregates. (C) SEM images of cell surfaces of MDA-MB-231. (D) Microscopy images of wound healing of MDA-MB-231 cells. Reproduced with permission [76]. Copyright 2017, American Chemical Society. | |
8. Conclusion and outlook
The responsive aggregation strategies have exhibited enhanced retention of nanoparticles in tumor sites and promoted anti-tumor efficacy to some extent. In addition to the enhanced retention and penetration in tumor sites, the aggregatable nanoparticles can also be used in other yields. Usually, the physicochemical properties of nanoparticles tend to be changed after the aggregation. For some metal nanoparticles, the aggregation shifts the surface plasmon resonance (SPR) to near-infrared region by the appearance of coupled SP mode, and endowed with photothermal and photodynamic effect for tumor treatment. Comparing with the direct modification of photo-sensitive molecules, the stimuli-triggered photothermal and photodynamic nanoparticles can protect peritumoral normal cells from damage. Moreover, the SPR shift could also lead to photoacoustic, PET and enhanced contrast of MRI [78], realizing the integration of diagnosis and treatment. As described in section 7, the aggregated nanoparticles could modulate tumor microenvironment in some way. The nanofiber-derived AECM could avoid tumor cells invasion and inhibit metastasis. And membraneadhered nanoaggregates could perturb the membrane and enhance influxof drugs. At cellular level, exocytosis of nanoparticles would be decreased after aggregation, which could be applied in multidrug resistance. There is great potential for stimuli-responsive nanoaggregates in many other fields which need further exploration.
Except for the size aggregation strategies, there is also a sizeshrinkage strategy to enhance both tumor accumulation and penetration. As a reverse mechanism compared with aggregation strategy, the size-shrinkage strategy first delivers relatively large nanoparticles to tumor sites, and then the original nanoparticles respond to some particular stimuli and shrink into small size for enhanced penetration. For now, both size aggregation and size shrinkage strategies exhibit both good tumor accumulation and penetration and show promoted anti-tumor efficiency. However, there is no proof to decide which strategy works better. Besides, they both have potential problems need to be fixed. As a result, there comes up with a size-reversible strategy, which integrated both advantagesof aggregation and shrinkage. Nanoparticles are designed to specifically respond to particular stimuli, and exhibit size reverse either from large to small and then to large or small to large and then to small [79, 80]. Though the current studies of size-reversible nanoparticles are not much, it has great potential in the future.
However, there are still a lot of challenges that need to be addressed in the future development. As we can see, the sizes of nanoparticles after aggregation are of great important, which should be large enough to accumulate in tumor sites and not to be pumped back to bloodstream. While it should be noted that the original sizes of nanoparticles before aggregation are equally important, which need to be carefully designed and balanced. If too large, it is difficult to get through vessels through EPR effect and penetrate deeply in tumor sites; if too small, they will be cleared up rapidly even before aggregation happens. Nanoparticles have been chosen for the stimuli-responsive aggregation for their easy modification and size controllability. While there are more and more research studies begin to question the system toxicity of nanoparticles. Long term accumulation of nanoparticles in normal tissues such as heart, kidney and liver will cause irreversible damage [81, 82]. In addition, recent studies found that nanoparticles would even promote cancer metastasis at low concentration [83, 84], which is against our original propose to inhibit tumor growth. Therefore, there is still a long way to go before clinical translation, but we look forward for the future progress and development.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No.81961138009), the Young Elite Scientists Sponsorship Program by CAST (No.2017QNR001), the Fundamental Research Funds for the Central Universities, 111 Project (No.B18035) and RFBR and National Natural Science Foundation of China Collaboration Project (No.19-58-55001).
| [1] |
W. Yu, R. Liu, Y. Zhou, H. Gao, ACS Cent. Sci. 6 (2020) 100-116. DOI:10.1021/acscentsci.9b01139 |
| [2] |
H. Gao, Curr. Drug Metab. 17 (2016) 731-736. DOI:10.2174/1389200217666160630203600 |
| [3] |
Q. Hu, Q. Chen, Z. Gu, Biomaterials 178 (2018) 546-558. DOI:10.1016/j.biomaterials.2018.03.056 |
| [4] |
S.M. Moghimi, A.C. Hunter, J.C. Murray, Pharmacol. Rev. 53 (2001) 283-318. |
| [5] |
C. Fang, B. Shi, Y. Pei, et al., Eur. J. Pharm. Sci. 27 (2006) 27-36. DOI:10.1016/j.ejps.2005.08.002 |
| [6] |
D. Liu, A. Mori, L. Huang, Biochim. Biophys. Acta 1104 (1992) 95-101. DOI:10.1016/0005-2736(92)90136-A |
| [7] |
H. Choi, W. Liu, P. Misra, et al., Nat. Biotechnol. 25 (2007) 1165-1170. DOI:10.1038/nbt1340 |
| [8] |
B. Shi, C. Fang, M.X. You, et al., Colloid Polym. Sci. 283 (2005) 954-967. DOI:10.1007/s00396-004-1243-8 |
| [9] |
G. Gao, Y. Li, D. Lee, J. Control. Release 169 (2013) 180-184. DOI:10.1016/j.jconrel.2012.11.012 |
| [10] |
A. Schadlich, H. Caysa, T. Mueller, et al., ACS Nano 5 (2011) 8710-8720. DOI:10.1021/nn2026353 |
| [11] |
X. Liu, Y. Chen, H. Li, et al., ACS Nano 7 (2013) 6244-6257. DOI:10.1021/nn402201w |
| [12] |
H. Cabral, Y. Matsumoto, K. Mizuno, et al., Nat. Nanotechnol. 6 (2011) 815-823. |
| [13] |
S. Kunjachan, R. Pola, F. Gremse, et al., Nano Lett. 14 (2014) 972-981. DOI:10.1021/nl404391r |
| [14] |
C. Li, J. Wang, Y. Wang, et al., Acta Pharm. Sin. B 9 (2019) 1145-1162. DOI:10.1016/j.apsb.2019.08.003 |
| [15] |
H. Liu, L. Chen, C. Xu, et al., Chem. Soc. Rev. 47 (2018) 7140-7180. DOI:10.1039/C7CS00862G |
| [16] |
Z. Gao, Y. Hou, J. Zeng, et al., Adv. Mater. 29 (2017) 1701095. DOI:10.1002/adma.201701095 |
| [17] |
L. Mei, J. Rao, Y. Liu, et al., J. Control. Release 292 (2018) 67-77. DOI:10.1016/j.jconrel.2018.04.053 |
| [18] |
G. Liang, H. Ren, J. Rao, Nat. Chem. 2 (2010) 54-60. DOI:10.1038/nchem.480 |
| [19] |
Y. Yuan, G. Liang, Org. Biomol. Chem. 12 (2014) 865-871. DOI:10.1039/C3OB41241E |
| [20] |
D. Ye, G. Liang, M. Ma, J. Rao, Angew. Chem. Int. Ed. 50 (2011) 2275-2279. DOI:10.1002/anie.201006140 |
| [21] |
Z. Zheng, P. Chen, G. Li, et al., Chem. Sci. 8 (2017) 214-222. DOI:10.1039/C6SC01461E |
| [22] |
W. Tang, J. Yang, Y. Yuan, et al., Nanoscale 9 (2017) 6529-6536. DOI:10.1039/C6NR09895A |
| [23] |
Z. Hai, J. Wu, D. Saimi, et al., Anal. Chem. 90 (2018) 1520-1524. DOI:10.1021/acs.analchem.7b05251 |
| [24] |
S. Ruan, R. Xie, L. Qin, et al., Nano Lett. 19 (2019) 8318-8332. DOI:10.1021/acs.nanolett.9b03968 |
| [25] |
S. Ruan, C. Hu, X. Tang, et al., ACS Nano 10 (2016) 10086-10098. DOI:10.1021/acsnano.6b05070 |
| [26] |
S. Ruan, W. Xiao, C. Hu, et al., ACS Appl. Mater. Inter. 9 (2017) 20348-20360. DOI:10.1021/acsami.7b02303 |
| [27] |
A. Dragulescu-Andrasi, G. Liang, J. Rao, Bioconjugate Chem 20 (2009) 1660-1666. DOI:10.1021/bc9002508 |
| [28] |
Y. Zhao, Z. Hai, H. Wang, L. Su, G. Liang, Anal. Chem. 90 (2018) 8732-8735. DOI:10.1021/acs.analchem.8b02704 |
| [29] |
J. Jeon, B. Shen, L. Xiong, et al., Bioconjugate. Chem. 23 (2012) 1902-1908. DOI:10.1021/bc300273m |
| [30] |
Y. Liu, Q. Miao, P. Zou, et al., Theranostics 5 (2015) 1058-1067. DOI:10.7150/thno.11758 |
| [31] |
B. Shen, J. Jeon, M. Palner, et al., Angew. Chem. Int. Ed. 52 (2013) 10511-10514. DOI:10.1002/anie.201303422 |
| [32] |
Y. Yuan, L. Wang, W. Du, et al., Angew. Chem. Int. Ed. 54 (2015) 9700-9704. DOI:10.1002/anie.201504329 |
| [33] |
D. Ye, A.J. Shuhendler, P. Pandit, et al., Chem. Sci. 4 (2014) 3845-3852. |
| [34] |
J. Gallo, N. Kamaly, I. Lavdas, et al., Angew. Chem. Int. Ed. 53 (2014) 9550-9554. DOI:10.1002/anie.201405442 |
| [35] |
Z. Wang, H.W. An, D. Hou, et al., Adv. Mater. 31 (2019) e1807175. DOI:10.1002/adma.201807175 |
| [36] |
C.E. Callmann, C.V. Barback, M.P. Thompson, et al., Adv. Mater. 27 (2015) 4611-4615. DOI:10.1002/adma.201501803 |
| [37] |
J. Zhou, X. Du, N. Yamagata, B. Xu, J. Am. Chem. Soc. 138 (2016) 3813-3823. DOI:10.1021/jacs.5b13541 |
| [38] |
Z. Zheng, P. Chen, M. Xie, et al., J. Am. Chem. Soc. 138 (2016) 11128-11131. DOI:10.1021/jacs.6b06903 |
| [39] |
Z. Feng, T. Zhang, H. Wang, B. Xu, Chem. Soc. Rev. 46 (2017) 6470-6479. DOI:10.1039/C7CS00472A |
| [40] |
Z. Feng, H. Wang, R. Zhou, J. Li, B. Xu, J. Am. Chem. Soc. 139 (2017) 3950-3953. DOI:10.1021/jacs.7b00070 |
| [41] |
L. Zhong, L. Xu, Y. Liu, et al., Acta Pharm. Sin. B 9 (2019) 397-409. DOI:10.1016/j.apsb.2018.11.006 |
| [42] |
S. Park, W.J. Lee, S. Park, et al., Sci. Rep. 9 (2019) 20180. DOI:10.1038/s41598-019-56754-8 |
| [43] |
X. Zhang, C. Zhang, M. Cheng, et al., Nano Res. 12 (2019) 2815-2826. DOI:10.1007/s12274-019-2518-1 |
| [44] |
Q. Guo, T. Lan, G. Wu, et al., Biomacromolecules 20 (2019) 3031-3040. DOI:10.1021/acs.biomac.9b00598 |
| [45] |
R.A. Mansbach, A.L. Ferguson, J. Phys. Chem. B 122 (2018) 10219-10236. DOI:10.1021/acs.jpcb.8b05781 |
| [46] |
P. Dogra, A. Joshi, A. Majumdar, S. Mukhopadhyay, J. Am. Chem. Soc. 141 (2019) 20380-20389. DOI:10.1021/jacs.9b10892 |
| [47] |
H. Li, Y. Chen, Z. Li, et al., Biomacromolecules 19 (2018) 2007-2013. DOI:10.1021/acs.biomac.8b00241 |
| [48] |
J. Nam, Y.S. Ha, S. Hwang, et al., Nanoscale 5 (2013) 10175-10178. DOI:10.1039/c3nr03698g |
| [49] |
D. Ni, D. Jiang, H.F. Valdovinos, et al., Nano Lett. 17 (2017) 3282-3289. DOI:10.1021/acs.nanolett.7b00995 |
| [50] |
Y. Zhou, R. Chen, H. Yang, et al., J. Mater. Chem. B Mater. Biol. Med. 8 (2020) 727-735. DOI:10.1039/C9TB02411E |
| [51] |
M. Zhou, H. Huang, D. Wang, et al., Nano Lett. 19 (2019) 3671-3675. DOI:10.1021/acs.nanolett.9b00737 |
| [52] |
F.C. Parks, Y. Liu, S. Debnath, et al., J. Am. Chem. Soc. 140 (2018) 17711-17723. DOI:10.1021/jacs.8b10538 |
| [53] |
E.W. Chan, S. Chattopadhaya, R.C. Panicker, X. Huang, S.Q. Yao, J. Am. Chem. Soc. 126 (2004) 14435-14446. DOI:10.1021/ja047044i |
| [54] |
L. Ming, L.Y. Gu, Q. Zhang, M.Z. Xue, Y.G. Liu, Chin. Chem. Lett. 24 (2013) 1014-1018. DOI:10.1016/j.cclet.2013.07.001 |
| [55] |
D. Manna, T. Udayabhaskararao, H. Zhao, R. Klajn, Angew. Chem. Int. Ed. 54 (2015) 12394-12397. DOI:10.1002/anie.201502419 |
| [56] |
X. Cheng, R. Sun, L. Yin, et al., Adv. Mater. 29 (2017) 1604894. DOI:10.1002/adma.201604894 |
| [57] |
Q. Pei, X. Hu, X. Zheng, et al., ACS Nano 12 (2018) 1630-1641. DOI:10.1021/acsnano.7b08219 |
| [58] |
W. Yu, X. He, Z. Yang, et al., Biomaterials 217 (2019) 119309. DOI:10.1016/j.biomaterials.2019.119309 |
| [59] |
Y. Lee, S. Lee, D.Y. Lee, et al., Angew. Chem. Int. Ed. 55 (2016) 10676-10680. DOI:10.1002/anie.201604858 |
| [60] |
R. Liu, M. Yu, X. Yang, et al., Adv. Funct. Mater. 29 (2019) 1808462. DOI:10.1002/adfm.201808462 |
| [61] |
J. Xu, L. Xu, C. Wang, et al., ACS Nano 11 (2017) 4463-4474. DOI:10.1021/acsnano.7b00715 |
| [62] |
B. Feng, B. Hou, Z. Xu, et al., Adv. Mater. 31 (2019) e1902960. DOI:10.1002/adma.201902960 |
| [63] |
L. Yang, P. Gao, Y. Huang, et al., Chin. Chem. Lett. 30 (2019) 1293-1296. DOI:10.1016/j.cclet.2019.03.032 |
| [64] |
T. Araki, Y. Fuchi, S. Murayama, et al., Nanomaterials Basel 8 (2018) 782. DOI:10.3390/nano8100782 |
| [65] |
Y. Deng, F. Kafer, T. Chen, et al., Small 14 (2018) e1802420. DOI:10.1002/smll.201802420 |
| [66] |
S.L. Qiao, Y. Ma, Y. Wang, et al., ACS Nano 11 (2017) 7301-7311. DOI:10.1021/acsnano.7b03375 |
| [67] |
M. Sun, D. Peng, H. Hao, et al., ACS Appl. Mater. Inter. 9 (2017) 10453-10460. DOI:10.1021/acsami.6b16408 |
| [68] |
T. Araki, S. Murayama, K. Usui, et al., Nano Lett. 17 (2017) 2397-2403. DOI:10.1021/acs.nanolett.6b05371 |
| [69] |
W.S. Cai, L.H. Gan, K.C. Tam, Colloid Polym. Sci. 279 (2001) 793-799. DOI:10.1007/s003960100506 |
| [70] |
M. Ebara, M. Yamato, T. Aoyagi, et al., Biomacromolecules 5 (2004) 505-510. DOI:10.1021/bm0343601 |
| [71] |
N.M. Matsumoto, G.W. Buchman, L.H. Rome, H.D. Maynard, Eur. Polym. J. 69 (2015) 532-539. DOI:10.1016/j.eurpolymj.2015.01.043 |
| [72] |
S. Yang, H. Gao, Pharmacol. Res. 126 (2017) 97-108. DOI:10.1016/j.phrs.2017.05.004 |
| [73] |
R. Kurzrock, D.J. Stewart, Clin. Cancer Res. 23 (2017) 1137-1148. DOI:10.1158/1078-0432.CCR-16-1968 |
| [74] |
W. Xiao, S. Ruan, W. Yu, et al., Mol. Pharm. 14 (2017) 3489-3498. DOI:10.1021/acs.molpharmaceut.7b00475 |
| [75] |
B. Li, Z. Jiang, D. Xie, Y. Wang, X. Lao, Int. J. Nanomedicine 13 (2018) 7289-7302. DOI:10.2147/IJN.S175334 |
| [76] |
X. Hu, P. He, G. Qi, et al., ACS Nano 11 (2017) 4086-4096. DOI:10.1021/acsnano.7b00781 |
| [77] |
X.X. Zhao, L.L. Li, Y. Zhao, et al., Angew. Chem. Int. Ed. 58 (2019) 15287-15294. DOI:10.1002/anie.201908185 |
| [78] |
S. Lu, X. Guo, M. Zou, et al., Adv. Healthc. Mater. (2019) e1901229. |
| [79] |
L. Yu, Y. Liu, S. Chen, Y. Guan, Y. Wang, Chin. Chem. Lett. 25 (2014) 389-396. DOI:10.1016/j.cclet.2013.12.014 |
| [80] |
Y. Su, T. Yu, W. Chiang, et al., Adv. Funct. Mater. 27 (2017) 1700056. DOI:10.1002/adfm.201700056 |
| [81] |
N.G. Bastus, E. Casals, S. Vazquez-Campos, V. Puntes, Nanotoxicology 2 (2008) 99-112. DOI:10.1080/17435390802217830 |
| [82] |
Y. Zhang, Y. Bai, J. Jia, et al., Chem. Soc. Rev. 43 (2014) 3762-3809. DOI:10.1039/C3CS60338E |
| [83] |
F. Peng, M.I. Setyawati, J.K. Tee, et al., Nat. Nanotechnol. 14 (2019) 279-286. |
| [84] |
M.I. Setyawati, C.Y. Tay, B.H. Bay, D.T. Leong, ACS Nano 11 (2017) 5020-5030. DOI:10.1021/acsnano.7b01744 |
 2020, Vol. 31
2020, Vol. 31 

