b Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen 518055, China
Cancer caused 9.6 million deaths all over the world in 2018 according to the estimation of GLOBOCAN [1]. The most widely used clinical treatments of cancer are chemotherapy, radiation therapy or surgery [2]. For chemotherapy, many chemical compounds and natural drugs have been discovered over the past several decades [3]. Most clinical anticancer drugs focus on disturbing nucleic acid synthesis (5-fluorouracil, methotrexate, cytarabine and mercaptopurine), damaging the structure and function of DNA (cyclophosphamide, bleomycin and cisplatin), interfering DNA transcription (dactinomycin and doxorubicin), or preventing protein synthesis (vinblastine and paclitaxel) [3-8]. The discovery of anticancer drugs provides hope for conquering cancer. However, tumoral selectivity of traditional chemotherapy drugs is not satisfactory enough. Traditional chemotherapies not only cause severe toxic side effects to patients, but also lack efficient anticancer output. To solve the undesired problems, drug delivery systems have been widely investigated since they were put forward in the 1950s, and now showing great potential in therapeutic effect [9]. The drug delivery systems can delivery drugs to desired sites through responding tumor microenvironment [3].
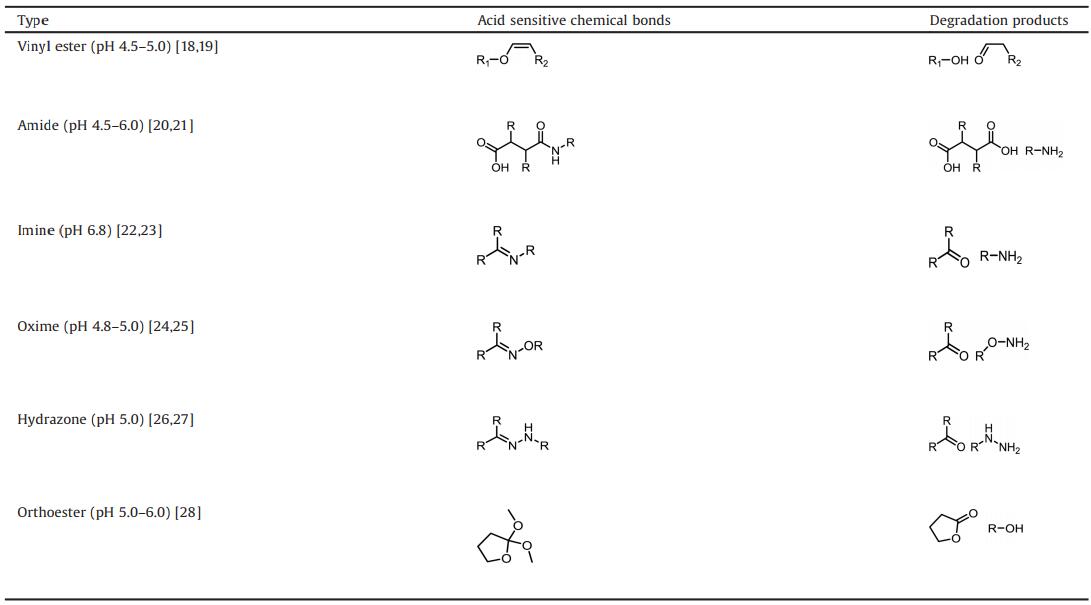
Distinct from normal cells, cancer cells have higher level of metabolization and faster proliferation, which makes tumor tissues form a unique microenvironment. The tumor microenvironment possesses several significant pathological characteristics, such as lower pH value, EPR effect (enhanced permeability and retention effect) and hypoxia [10]. pH value of tumor microenvironment is one of the most widely investigated features. Due to faster glycolysis and hypoxia condition occurring in solid tumors, solid tumor usually produces large amount of lactic acid from glucose to provide enough energy for tumor growth. The exclusion of H+ from tumor cells contributes to acid tumor extracellular microenvironment. The lower pH tumor microenvironment exists in almost all of the solid tumors, regardless of tumor development periods or tumor types [11]. As shown in Fig.1, the extracellular pH of tumor cells is around 6.0, while extracellular pH of normal cells is around 7. 4. Meanwhile, the intracellular pH of the tumor cells is a little higher than that of normal cells, but the pH of lysosome in tumor cells is lower than that of normal cells.

|
Download:
|
| Fig. 1. Schematic comparation of the pH differenceon cellular level. | |
Based on the pH difference between cancer and normal tissues, various novel drug delivery systems are fabricated to enhance the selectivity of traditional chemotherapeutic drugs [12]. These systems smartly deliver drugs upon responding to acid tumor microenvironment, which guarantees the cancer inhibitory efficacy and reduces systemic toxicity. The fabrication of the pH-sensitive drug delivery systems is inseparable from the material science. Many novel pH-responsive nanoscale materials have been developed in the last decades. Their properties could be changed while meeting with different pH environment. Part of these nanoscale materials can change their physical properties like shape, size or rigidity in specific pH range. Part of them can change their chemical properties in the low pH such as hydrophilicity/ hydrophobicity, isomerization, or surface charge. The others even conduct chemical reactions under acidic environment [13, 14]. pH-sensitive nanoscale materials like ionizable polymers and copolymers, linker-based polymers, metal organic frameworks, inorganic nanoparticles and biomolecular materials are commonly used for pH-sensitive drug delivery to enhance the anticancer therapeutic efficacy while decreasing systemic toxicity [15-17]. The mechanisms of pH-sensitive drug delivery systems mainly include pH-sensitive bond cleavage and protonation mechanism, which are theoretical basis for designing smart and responsive drug delivery systems. To date, combination therapy has revealed additive and even synergistic anti-cancer efficacy. Various novel materials that are not only pH sensitive but also have other extra properties beneficial for anticancer treatment are employed to realize such combination therapy.
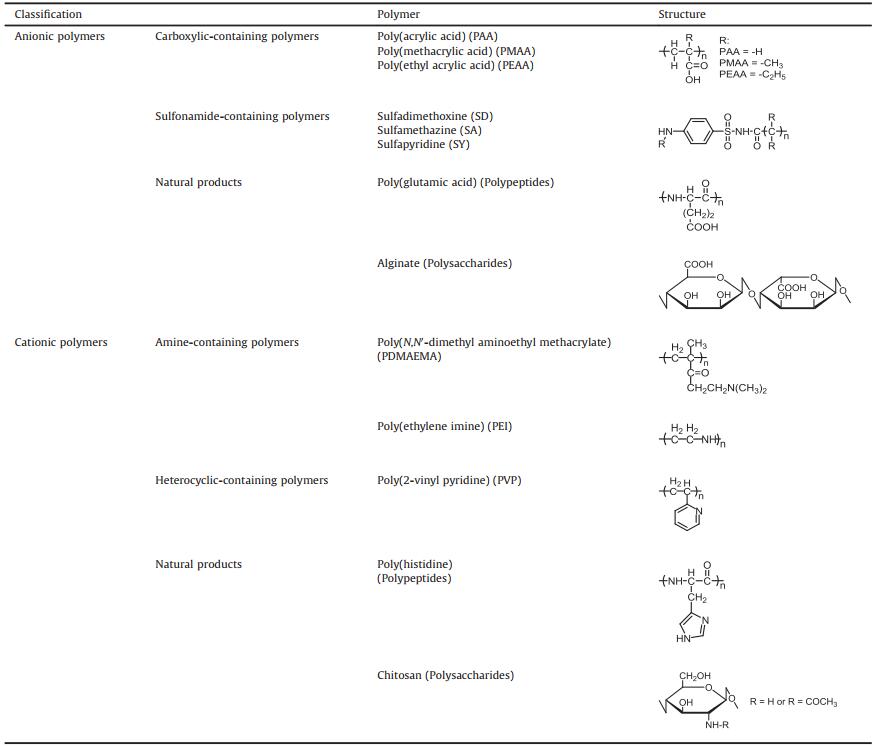
There are lots of reviews that introduce smart drug delivery systems. In this review, we mainly focus on the drug delivery systems that are pH sensitive and enhance anticancer output especially in acidic tumor microenvironment. We summarized traditional and newly applied pH-sensitive nanomaterials through their categories: polymers, inorganic materials and biomaterials, which are shown in Fig.2.
|
Download:
|
| Fig. 2. pH-Sensitive nanomaterials. | |
2. Mechanism of pH-sensitive drug delivery systems 2.1. Mechanisms of pH sensitivity of drug delivery systems
pH-Sensitive drug delivery systems can deliver anticancer drugs under acidic tumor microenvironment. Commonly, there are two mechanisms that explain the essence of pH-sensitivity of systems: acid sensitive chemical bond cleavage and protonation of materials. Though most of the pH-sensitive drug delivery systems can be explained through these two mechanisms, some novel delivery systems are based on their unique mechanisms.
2.1.1. pH-Sensitive chemical bond cleavageThe stability of pH-sensitive chemical bonds usually varies with pH variation. Some of pH-sensitive chemical bonds are stable under physiological pH environment (around 7. 4) but are cleaved under acid tumor microenvironment. This type of pH-sensitive chemical bonds, also named pH-sensitive linkers, can be designed to form drug delivery materials or combine drugs with materials. Based on pH-sensitive chemical bonds, these nanoscale delivery systems could release drugs by cleavage of linkers that anchor drugs on material or by disassembly of materials. There are many types of pH-sensitive chemical bonds, each of which is different in reaction rates and reaction pH value. The diversity provides more options for drug delivery to specific tumor accurately. The most popular investigated pH-sensitive chemical bonds in cancer therapy and their degradation products are shown in Table 1, which include vinylesters, amides, imines, oximes, hydrazones and orthoesters.
|
|
Table 1 Selected examples of pH-sensitive chemical bonds and their degradation products. |
Vinyl ether bond linkers are sensitive to pH 4.5–5.0, which is due to degradation of hemiacetal intermediate through the β carbon protonation of vinyl ether bond. The protonation and degradation processes are the rate determining step of the bond cleavage reaction, which is tunable through changing substituents around the esters [14, 29].
β-Carboxylic amides are substituted amides and they possess similar reaction process with esters, which are commonly served as amide linkers. β-Carboxylic amides are used for charge reversal due to the unique process, more specifically, the β-carboxylic amides sustain negative charged under physiological pH whereas transfer to positive charged primary amines under tumor acidic pH [20]. The substituted amides linkers are sensitive to pH 4.5–6.0 depending on the chemical property of the substituted groups.
Imines, oximes and hydrazones all have the labile structure of C=N, the stability of the C=N structure is because π electrons participating inthe delocalization effect. Imine linkers are especially sensitive to weak acid environment (pH around 6. 8), however, they are too sensitive to keep stable in physiological environment. Oxime linkers hold better thermodynamic stability, hence they are stable in physiological environment and can release drug slowly.
Hydrazones linkers are more commonly used because they are balanced in stability and sensitivity. They are sensitive to pH around 5.0, which is more suitable for distinguishing pathological conditions from physiological conditions [14]. Besides, some of hydrazine linkers based systems have undergone clinical trials [14, 30]. The pH-sensitivity and stability of these linkers can be tuned through change the substituents, for instance, benzoic imine linkers are more stable and exhibit desirable release behaviors [31].
2.1.2. Protonation of materialsDifferent from cleavage of pH-sensitive chemical bonds, the protonation mechanism refers to the materials that can be protonated under acidic tumor microenvironment. Protonation of nanoscale materials usually causes the change of material properties, like stability, surface charge and morphology. For example, cationic polymers could expand after protonation under acidic conditions, thus specially releasing buried drugs in tumor tissue. Materials such as pH sensitive polymers, peptides and inorganic materials (include metal and nonmetal), are used to fabricate protonation mechanismbased pH-sensitive drug delivery systems [13], each of which exists different properties and will be discussed in the following part.
2.2. Mechanisms of pH-sensitive nanomaterials enhanced anticancer efficacypH-Sensitive drug delivery systems could target acidic tumor microenvironment and enhance the anticancer effects especially under the functions of lower pH condition. Most of mechanisms of pH-sensitive nanomaterials can enhance anticancer efficacy owing to pH enhanced cell uptake or pH enhanced drug release.
To reduce systemic toxicity of drug delivery systems, the nanomaterials should deliver drugs to tumor tissue and release drugs especially in tumor cells or tumor microenvironment. On the one hand, nanomaterials of drug delivery systems should avoid being cleaned by reticulo-endothelial system (RES) and kidney. Whereas the systems should efficiently accumulate in tumor tissue. The requirements indicate that nanomaterials for drug delivery systems should satisfy several features, like negative surface charge and 20-100 nm size. However, the features beneficial for long-time circulation also impede the tumor cell uptake of nanomaterials. To solve the dilemma, the pH-sensitive nanomaterials that could change their surface charge or morphology are designed, which could especially enhance cell uptake of anticancer drugs in acidic tumor microenvironment. The common Ph enhanced cell uptake strategies are charge reversal strategy and morphology change strategy. On the other hand, the nanomaterials of drug delivery systems should avoid releasing drug until reaching tumor tissues or entering tumor cells. The common pH enhanced drug release strategies are nanostructure degradation strategy, bond cleavage strategy and gatekeeper strategy [32-34].
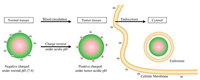
Charge reversal strategy is based on charge switchable nanoparticles, and these nanoparticles can reverse their surface charge under specific condition, which changes the electrostatic interactions between the nanoparticles and serum, cellular membrane and organelles [35]. Positively charged nanoparticles are more likely to be endocytosed by cells because cellular membrane is negatively charged, but these nanoparticles are also easily be cleaned by RES after administration [20]. Negatively charged nanoparticles are also imperfect. Though the RES clearance of negatively charged nanoparticles is low, their cell uptake efficacy is not satisfying. For this reason, charge reversal strategy is a better option to balance the charge dilemma. Generally, the nanoparticles are negatively charged in blood circulation, and they are stimulated by tumor lower pH microenvironment and subsequently reverse to positively charged nanoparticles which show enhanced cellular uptake in tumor site, the schematic illustration is shown in Fig.3 [36].
|
Download:
|
| Fig. 3. The mechanism of charge-reversal enhanced cellular uptake. | |
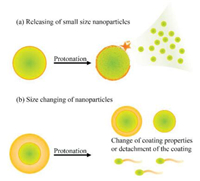
The morphology of the nanoparticles includes particle size and shape, which also influences the distribution, penetration, pharmacokinetic and cellular uptake of the nanoparticles [32, 34]. The smaller nanoparticles possess deeper penetration ability but lack of duration, whereas larger nanoparticles (10-200 nm) possess longer duration ability but lack of penetration. Therefore, size changeable drug delivery systems are also thought to a solution for size dilemma, Fig.4 gives examples of such size changeable systems. Some of systems can release encapsulated small nanoparticles after protonation, and some of systems can change their size after protonation. Such systems are commonly prepared through coating or self-assembling.
|
Download:
|
| Fig. 4. Examples for size changeable systems. (a) Releasing of small size nanoparticles: the systems release encapsulated small particles after protonation; (b) Size changing of nanoparticles: the systems are changed in size after protonation, coating or self-assembly size changeable systems are generally designed in this manner. | |
Nanostructure degradation strategy is the wildest employed drug releasing manner. In the process, nanostructure of drug delivery systems breaks down under acidic tumor microenvironment, thus leading to drug release in tumor site [10, 37]. For example, CaCO3 nanoparticle is decomposed by H+ under acid condition and releases the buried drugs.
Bond cleavage strategy is also a common method to release drug response to acidic tumor microenvironment. The mechanisms of bond cleavage strategy have been discussed before. And the drug will be released from carriers through bond cleavage reaction triggered by acid tumor microenvironment.
Gatekeeper strategy is a kind of on-demand drug release strategy. The gatekeeper systems mainly consist of mesoporous materials and pH-sensitive door. Mesoporous materials carrying drugs serve as core and pH-sensitive materials act as "gatekeeper" on the surface [31, 38]. The "gatekeepers" mainly prevent drug from releasing in physiological environment and induce drug release under acidic conditions specially. Mesoporous silica nanomaterial (MSN) is the most commonly used core material; and metal oxides, pH-sensitive polymers, pH-sensitive peptides and some pH-sensitive bonds are often employed as "gatekeeper". An example of such gatekeeper based drug delivery systems is showed in Fig.5. The DOX loaded systems were able to release through PEG detachment triggered by acidic environment.

|
Download:
|
| Fig. 5. An example for schematic illustration of gatekeeper based pH-sensitive drug delivery strategy. (a) The dynamic interaction of the pH-sensitive bond. (b) Drug release progress under acidic environment. (c) Cellular uptake and site-specific drug release of the gatekeeper strategy. Copied with permission [31]. Copyright 2017, Wiley. | |
3. pH-Sensitive polymers
Based on protonation mechanism, polymers containing ionizable pendant groups can be designed to pH-sensitive drug delivery systems, the ionic functional groups mainly include weak acid (anionic polymers) and weak base (cationic polymers), and the examples of anionic and cationic polymers are shown in Table 2. These functional groups can accept or donate proton (H+) while pH is changing. This progress is also called protonation or deprotonation, which influences the ionization degree and net charge of molecules. With the change of ionization degree and net charge, the solubility, hydrophilicity/hydrophobicity and other properties of polymers changed, which leads to the variation of morphology, hydrodynamic size and chain conformation of the polymers [39, 40]. For example, molecular chains of some polymers transit from collapsed state to expanded state while electrostatic repulsion increases [39], which increases the hydrodynamic size and inter space of polymers. On the other hand, acid degradable copolymers containing pH-sensitive chemical bonds are also employed to realize pH-sensitive drug delivery, such as polydopamine (PDA) [41].
|
|
Table 2 Selected examples of anionic and cationic pH-sensitive polymers. |
3.1. Anionic polymers
Some anionic polymers contain anionic functional groups such as carboxylic acids and sulfonamides, which can be obtained from chemical synthesis or natural products, such as polysaccharides and polypeptide.
Carboxylic acid (-COOH) is a kind of weak acid, and it partially ionizes to -COO- in aqueous solution. In normal pH environment (low H+ concentration), most of carboxylic groups on the polymer are in -COO- form, while encountering acidic pH environment, the carboxylic groups are protonated to -COOH form, therefore, the charge changing leads to the properties change and causes drug release [39, 42]. The most commonly investigated anionic polymers are poly(acrylic acid) (PAA), poly(methacrylic acid) (PMAA) and poly(ethyl acrylic acid) (PEAA). Alkyl side groups are decorated onto the chain to regulate the pH-sensitivity. The hydrophobicity of the molecule is enhanced with the increasing of alkyl side groups, and the higher hydrophobicity leads to higher pKa of the polymer. As a result, the pKa values of PAA and its alkyl derivatives are different: PAA (4.28) [43], PMAA (6.3), PEAA (6.7) [44].
Sulfonamides are a series of molecule composed by p-amino-benzenesulfonamide (Ar-SO2-NH-R), and there are similarities between the mechanism of carboxylic acid and sulfonamides based polymer. In the sulfonamides, the sulfonyl group serves as electron-withdrawing functional group and causes deprotonation of RNH-. This kind of chemical equilibrium is pH-sensitive, and the pKa is adjustable from 3 to 11 through varying substituting group beside the SO2NH group [14, 39, 45].
Many natural products can be unitized as monomers to synthesize polymers under specific condition. In this field, polysaccharides and polypeptides are the most popularly researched polymer. Besides, some metabolic intermediates can also serve as monomers to form anionic polymers, like polydopamine synthesized from dopamine. Polypeptides are polymerized from amino acid monomers via amide bonds. The pH-sensitivity is dependent on ionizable pendant groups on the polypeptide chain. Glutamic acid is ionizable because the presence of carboxylic groups. Zheng et al. [46] have fabricated a poly(L-glutamic acid) grafted mesoporous silica nanoparticles for pH-sensitive doxorubicin (DOX) delivery, which exhibited high drug loading efficiency and released DOX much more quickly in pH 5.5 than in pH 7.4. As a result, the nanoparticles showed enhanced anti-cancer effect than that of traditional DOX. Polysaccharide naturally exists in some plants. It has widely applied in pharmaceutical excipients [39]. Alginate is a kind of biomaterial which is generally extracted from brown algae (Phaeophyceae), the presence of carboxylic groups endows the pH-sensitivity for the alginate [47].
3.2. Cationic polymersCompared with anionic polymers, cationic polymers undergo opposite protonation or deprotonation process when the pH environment is changing. Cationic polymers mainly include amine-containing polymers and heterocyclic-containing polymers, and natural products-based polymers (polypeptides and polysaccharide)
Amines (RNH2; RNHR'; RN(R')R") are alkyl substituted functional groups and are important compositions of organic weak bases. The amine-containing polymers have ionizable primary, secondary or tertiary amine groups decorated on their side chains, or have a secondary amine in the main chain. These polymers can be positively ionized under low pH environment. The widely used amine groups are: acrylamide, aminoethyl methacrylate, vinylamine, N, N'-dimethyl aminoethyl methacrylate and N, N'-diethyl aminoethyl methacrylate [15].
Poly(N, N'-dimethyl aminoethylmethacrylate) (PDMAEMA) and poly(N, N'-diethyl aminoethyl methacrylate) (PDEAEMA) are the typical side chain amine-containing polymers. The pH-sensitivity of these types of polymers can be enhanced through increasing the number of ionic groups on the side chains. The increased ionic groups cause decreased hydrophobic interactions, and finally make the polymers more likely to be protonated [15, 39].
Poly(ethylene imine) (PEI) is a linear imine containing highly charged polymer. PEI possesses strong proton accepting ability in the low pH environment, owing to the -NH- groups that are directly located in the polymer main chain [48]. The strong proton accepting ability is suitable for delivery of gene therapeutic drugs. However, cationic polymers, especially PEI, are more toxic than anionic materials.
Heterocyclic functional groups containing N atoms can serve as pH-sensitive groups, because N atom owns a lone pair of electrons that are able to accept protonsin acid environment. The heterocyclic functional groups are protonated to positively charge aromatic ion under the acid environment. Therefore, N atom containing heterocyclic groups possess the similar weak alkaline properties with amine, which can be decorated to the side chain of the polymers.
The most commonly employed heterocyclic functional groups are pyridine and imidazole. The most popular pyridine groups containing polymer are poly(2-vinyl pyridine) (pKa around 5.9) and poly(4-vinyl pyridine) (pKa around 5. 4), and imidazole containing polymers are poly(N-vinyl imidazole) and poly(4-vinyl imidazole) (both pKa around 6.0) [39].
Peptides containing base groups such as histidine, arginine and lysine, are polymerized to cationic polypeptides. Poly(histidine), poly(arginine) and poly(L-lysine) are the widest investigated cationic polymers, the pKa of them are 6.0, 10.5 and 12.5, respectively [39].
Chitosan is a cationic polysaccharide prepared from chitin through N-deacetylation, which has good biocompatibility and low toxicity, and has been used for drug delivery in recent years [49]. Chitosan consists of D-glucosamine and N-acetyl-D-glucosamine units, and deacetylation degree is the key of properties of the pH-sensitivity. Chitosan precipitates in basic pH environment and dissolve in acidic environment, this unique pH-response behavior is utilized for pH-sensitive drug delivery [49, 50].
3.3. CopolymersPolymers prepared from single type of monomer cannot meet the demand of precise drug delivery. Recently, copolymers prepared from two or more types of monomers have been popularly investigated. They contain different kinds of structural units that endow the copolymers multi-properties to realize precise drug delivery. In this stage, amphiphilic diblock copolymer and acid sensitive linker-based copolymer are most commonly used to fabricate multi-functional pH-sensitive drug delivery systems.
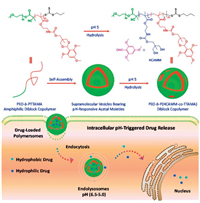
Amphiphilic diblock copolymers contain both hydrophilic structure and hydrophobic structure. Many of amphiphilic diblock copolymers can self-assemble spontaneously in aqueous solution and form micelle or polymersome to encapsulate anti-cancer drugs. Some structures are sensitive to acidic environment, that is, they can response to the pH variation and change their properties (e. g., solubility, hydrophobicity, net charge and shape) or produce chemical reactions. These structures are commonly used to synthesize amphiphilic copolymer with other structures. When copolymers encounter specific pH environment, the pH-sensitive chemical structures change and affect the properties of whole copolymer and finally induce drug release through structure disintegration or bond cleavage [52]. Wang et al. [51] reported an acid disintegrable polymersome of pH-sensitive amphiphilic diblock copolymers, poly(ethyleneoxide)-b-poly(2-((((5-methyl-2-(2,4,6-trimethoxyphenyl)-1,3-dioxan-5-yl)methoxy)carbonyl) amino)ethyl methacrylate) (PEO-b-PTTAMA). The copolymers were prepared by addition–fragmentation chain transfer (RAFT) polymerization of TTAMA (as the pH-sensitive monomer) with PEO-based RAFT agent. As shown in Fig.6, the polymersome had pH-responsive hydrophobic and hydrophilic bilayers structure, which possessed ability to load hydrophobic and drugs simultaneously. When the PTTAMA encountered with acidic pH environment (around 5), structure would hydrolyze to form pore on the polymersome and result in drug release, which showed good pH-sensitive effect both in vivo and in vitro. Yang et al. [53] have fabricated a kind of dextran-g-poly(histidine) copolymer to encapsulate immunotherapy agents CSF-1R inhibitor for cancer therapy, the system exhibited excellent pH-sensitivity and enhanced immune effect. Yu et al. [54] have prepared pillar[5] arene-based amphiphilic brush copolymer to realize self-imaging targeted drug delivery, the copolymer could response to low pH and reductase to release drug and generate fluorescence, which combined diagnosis and treatment.
|
Download:
|
| Fig. 6. An example for amphiphilic diblock copolymers as pH-sensitive drug delivery systems. Copied with permission [51]. Copyright 2015, American Chemical Society. | |
Acid sensitive linkers-based copolymers contain pH-sensitive chemical bonds that have discussed before. The chemical bond in copolymers can be cleaved under acidic pH environment, which causes drugs to directly release from carrier or system. Zhang et al. [55] have developed a kind of acid sensitive DOX copolymer prodrug nanoparticles. The copolymer was PMABH2-b-PEOGMA-FA and linked with DOX via pH-sensitive hydrazone bond. It could form micelle and encapsulate IR825 (a NIR absorbing dye) through self-assembling in aqueous solution. The prepared micelles were responsible for dual responsive mechanism:hydrazone bonds were pH-sensitive for releasing DOX in acidic tumor microenvironment, FA fragments were FA receptor targeting for receptor mediated endocytosis. Huang et al. [56] have designed a similar prodrug DOX micelle. the copolymer was designed as mPEG-b-PAE-cis-DOX, the PAE fragment and amide bond linked DOX were pH-sensitive, which realized better pH-responsibility.
3.4. Metal organic frameworksVarying in shape, size, and function, metal organic frameworks (MOFs) are prepared via linking inorganic materials and organic polymers through strong bonds (reticular synthesis), which have attracted interests of researchers since they were discovered in 1989 by Robson [57, 58]. MOFs have many special features, for example, the porosity of MOF structure allows for encapsulating large molecules, proteins, and drugs stably; MOFs have combined the properties of metal and polymer to realize multi-responsibility; the MOFs have better biocompatibility and lower toxicity [16]. In the recent decade, MOFs have been popularly investigated in drug delivery, catalyzation, material and energy science areas. To date, the trending of MOFs researches is still on the rise.
Zeolitic imidazolate framework (ZIF), UiO-66 and MIL-100 are the most commonly used pH-sensitive MOF materials, which have permanent porosity, high thermal and hydrothermal stabilities [59-64]. Zheng et al. [59] have synthesized DOX-encapsulated ZIF-8 nanoparticles through one-pot method for pH-responsive drug delivery. The ZIF-8 nanoparticles exhibited excellent pH response, zero-release was observed at pH 6.5 whereas full-release was observed at pH 6.0. Wang et al. [65] have fabricated MIL-100 based nanoparticles for chemo/photothermal combinational cancer therapy. The nanoparticles were able to serve as magnetic resonance imaging contrast and fluorescence optical imaging (FOI) agent to realize dual-model imaging guided chemo/photothermal therapy to treat cancer.
Polydopamine (PDA) has strong adhesive ability, which is synthesized from catecholic amino acids dopamine, can surfacefunctionalize with various materials, such as ceramics, metal materials, semiconductors, and some polymers [41, 66]. Owning to its good adhesivity, biocompatibility and photothermal ability, PDA has been popularly investigated for MOFs in recent decades. Wu et al. [67] have fabricated PDA coated ZIF-8 nanoparticles that could respond pH and near-infrared (NIR) to deliver anticancer drug, which showed enhanced anticancer effect.
Some transition metal materials, which are able to response photo or magnet for imaging in vivo, or able to generate photodynamic or photothermal effects, are generally fabricated to MOFs to decrease the toxicity and obtain multi-functions.
4. pH-Sensitive inorganic materialsIn addition to organic polymers, many inorganic materials, for example, calcium nanoparticle, metal oxides and graphene oxides, also have pH-sensitivity. Inorganic materials have their unique pH-sensitive mechanism. Calcium materials and metal oxides (iron oxides, manganese oxides and zinc oxides) are acid soluble to deliver drug. Other materials such as graphene oxides, gold nanoparticles, black phosphorous and mesoporous silica, have particular covalent and non-covalent interactions that can be changed by protonation, and changing of interactions triggered by pH is utilized to deliver drug. Moreover, there are different kinds of preparation method in some inorganic materials, which is available for fabricating nanoparticles in various shapes (particle or layer).
4.1. Calciumcarbonate (CaCO3) and calcium phosphate (CaP)Many calcium salts such as calcium carbonate (CaCO3) and calcium phosphate (Ca(H2PO4)2 and Ca3(PO4)2) can react with H+ and degrade. This property is employed to design pH-sensitive drug delivery systems. The calcium salts show promising potential in pH-sensitive drug delivery because of their biocompatibility, slow biodegradability, safety and availability. Moreover, the gene delivery through co-precipitation of calcium ion with nucleic acid is a highlight of calcium salt nanoparticles [68].
In anticancer drug delivery, the calcium nanoparticles can be endocytosed via cellular uptake and then encapsulated into acidic lysosome, finally degraded to calcium ion. An increased calcium level in the cytosol is harmful to cancer cell [69], which might exert synergistic effect with chemotherapy drugs.
Calcium carbonate (CaCO3) is widely existing in the nature, however, the natural CaCO3 cannot be employed as drug carrier directly. To deliver drugs, anhydrous crystalline is commonly used. The anhydrous crystalline polymorphs of CaCO3 are calcite (rhomboidal), aragonite (needle-like) and vaterite (spherical). The CaCO3 morphological forms are determined by preparation method and conditions (e. g., reactants, temperature and additives) [68]. The generally used methods to prepare CaCO3 nanoparticles are emulsion method and chemical precipitation method [70, 71]. In the emulsion method, the microemulsion droplets of water and oil phase form reaction environment, the ions can transport to other phase to form CaCO3 nanoparticles. In the chemical precipitation method, the CaCO3 nanoparticles are prepared through the reaction between calcium ions (e. g., calcium chloride or calcium nitrate) and carbonate ions (e. g., sodium carbonate). However, the particle size and polymer dispersity index of the CaCO3 nanoparticles are hard to control in this method [71, 72].
The degradation properties of CaCO3 nanoparticles is ideal for tumoral pH-sensitive drug delivery. The CaCO3 nanoparticles hardly degrade in physiological pH environment (7.4), while rapidly degrade in tumor acidic microenvironment (pH < 6.5) [68]. Moreover, the biological safety of the CaCO3 nanoparticles on HeLa cells was also evaluated by Zhang et al. [73], which showed that CaCO3 nanoparticles could serve as relatively safe and biocompatible drug carrier.
Som et al. [74] have prepared monodispersed pH-sensitive calcium carbonate nanoparticles for modulating tumor local pH and inhibiting tumor growth in vivo. The nanoparticle could selectively accumulate in tumor site, modulate the abnormal pH environment and inhibit the tumor growth. Zhou et al. [75] have developed an aptamer CaCO3 nanostructure with a high drugloading capacity as an anticancer theranostic system, the DOX was loaded into the CaCO3 nanostructure and biotin aptamer was modified on the surface of the nanostructure. The aptamer enhanced cellular uptake of the anticancer system through receptor-mediated endocytosis, and CaCO3 core responded to the lysosomal pH (around 4.5–5.5). Both of two mechanisms realized synergistical anti-cancer effect. Dong et al. [17] have reported Ce6Mn (a photosensitizer) and DOX co-loaded CaCO3 nanoparticles. This nanoparticle enabled real-time drug release monitoring via magnetic resonance (MR) imaging and realized combination antitumor therapy of chemotherapy and photodynamic therapy (PDT).
Calcium phosphate is a series of calcium orthophosphate compounds, in most cases, the Ca to P molar ratio varies from 0.5 to 2 [76-78]. Human bone is known to be composed of 70% CaP [77], therefore CaP is a kind of safe and biocompatible material widely used in biomedical applications, such as orthopedic and dental operations, bone reparation, and more recently, drug delivery. The CaP holds similar pH-sensitivity to CaCO3 and its amorphous crystalline is commonly used. There are many double decomposition based preparation methods to obtain CaP nanoparticles [79], such as wet chemical precipitation method [80], co-precipitation method [33], and micro-emulsion method [81].
Nucleic acid delivery is one of the most special properties of calcium material, which is a general approach to gene therapy. Li et al. [81] have prepared a kind of lipid coated calcium phosphate nanoparticle to realize siRNA delivery. To prepare such system, the siRNA was co-precipitated with CaP core in alkaline condition (pH around 9) and finally coated by DOTAP to form liposome-like lipids coated calcium phosphate nanoparticle. The nanoparticle entered cell and de-assembled in low pH endosome, and finally released entrapped siRNA to exert anti-cancer effect.
In recent years, owning to nice compatibility of CaP, foreign substances doped CaP is popularly investigated to combinate diverse properties. Fu et al. [82] have fabricated a biodegradable manganese-doped pH-sensitive CaP nanoparticle for traceable cascade antitumor theranostics. The CaP systems consisted of glucose oxidase (GOx), DOX and Mn2+, as GOx realized starvation therapy through eff; ectively eliminating intratumor glucose, Mn2+ caused chemodynamic therapy through toxic hydroxyl radicals that are generated by Fenton-like reaction, and DOX mediated chemotherapy. Besides, the enzyme activity kept stable for a long time because the CaP had served as a high compatible and stable carrier for the GOx, the CaP had shown prominent application in enzyme delivery.
4.2. Iron oxidesIron oxides include FeO, Fe2O3 and Fe3O4 (FeO·Fe2O3), their acid solubility is used for pH-sensitivity drug delivery. Thereinto, the Fe3O4 can be prepared as superparamagnetic iron-oxide nanoparticles (SPION), which endows pH and magnetic dual responsibility for the drug delivery systems [83]. Besides, iron oxides have been investigated broadly and evaluated clinically. FDA had approved iron oxide nanoparticle ferumoxytol injection in 2009 [84], Zanganeh et al. [85] have found that ferumoxytol could inhibit tumor growth through inducing pro-inflammatory macrophage polarization. This work might provide iron oxides other new theoretical basis for cancer therapy.
Iron oxides also exhibit good compatibility with other materials, recently, various iron-hydride nanostructures have been fabricated to satisfy complicated tumor environment. Xu et al. [86] have designed Fe3O4-Salphen nanoparticles for killing cancer cells selectively, the composite nanoparticles are combined with intense photoluminescence, magnetic responsiveness and pH-sensitivity, which showed enhanced tumor killing effect. Wu et al. [87] have fabricated ultrasmall iron oxide nanoparticle contained MSNs theranostic platform. The highly dispersed Fe nanoparticles were included in mesopore channels to exert nontoxic contrast agents for MRI (magnetic resonance imaging), and DOX were linked with Fe ion to realize chemotherapy. This system was pH-sensitive and showed capability in solving multidrug resistance (MDR) problem of cancer cells. Lu et al. [88] have developed pH-sensitive iron oxide nanocluster assemblies to diagnose hepatocellular cancer. The system provided inverse contrast enhancement effect to obtain distinction between normal cells and cancer cells.
4.3. Manganese oxidesManganese oxides have acid sensitivity and magnetic resonance effect, which is similar to iron oxides. The advantages of manganese oxides are, they have a high-surface-area and shape controllable [89]. Chen et al. [90] have prepared a kind of 2D MnO2 nanosheets for pH-sensitive cancer theranostics, the MnO2 nanosheets were exfoliated from HMnO2 and PEGylated. The nanosheets were used for diagnosis through pH-responsive MRI and drug delivery through pH-responsive drug release, and finally induced cell apoptosis to exert anticancer effect. Liu et al. [91] have fabricated multi-sensitive (redox, pH and H2O2) MnO2 nanoparticles to realize cancer theranostic, which showed better antitumor effect owning to its multi-sensitivity. Hsu et al. [92] designed MnO-based bimodal nanoprobe for cancer imaging, which was pH-activatable fluorescence and magnetic resonance nanoprobe. MnO nanoparticles served as fluorescence quencher for coumarin-545 T before cellular uptake, whereas fluorescence recovery was achieved through acidmediated MnO disintegration. Moreover, the released Mn ions could be employed as contrast agents for MRI. Kim et al. [89] have obtained urchin-shaped manganese oxide nanoparticles for pH-sensitive tumoral MRI, the urchin shape endowed the system high-surface-area for unloading the cargo.
4.4. Zinc oxidesZinc oxide (ZnO) nanoparticles not only rapidly dissolve in tumor acid pH environment (pH < 5.5) [93], but also hold inherent anticancer effects. The molecular mechanism is, ZnO nanoparticles are able to selectively induce cellular apoptosis through generating reactive oxygen species (ROS) in cancer cells [94], which is promising for exerting synergistic anticancer effect. Cai et al. [95] have proposed pH-sensitive ZnO nanocluster for lung cancer drug delivery, which was composed of DOX-loaded ZnO core and PEG cluster. After being endocytosed by cancer cells, the nanoclusters escaped from endosome and released the loaded drug to realize anticancer effect. Wang et al. [96] have prepared multitarget and multifunctional ZnO nanoparticles for pH-sensitive drug delivery, macrophage polarization and cancer stem cell CD44 expression down regulation, the nanoparticles exhibited good anticancer effect owning to multi-responsibility.
4.5. Other inorganic materials 4.5.1. Graphene oxidesHolding two-dimensional plane structure, graphene consists of a thick layer of carbon atoms, which has extraordinary wide surface area and large π conjugation [97]. Graphene oxides (GO) that own oxygen-containing active groups (e. g., -OH, -C = O, -COOH) are the oxide species of graphene, which provides numerous reaction sites for surface modification [98].
The GO is usually employed as a carrier for drug delivery systems in recent decade. On the one hand, the oxygen-containing groups are generally designed to form pH-sensitive chemical bonds mentioned before so as to realize drug loading. On the other hand, non-covalent interactions like π-π stacking [99, 100], Van der Waals force [101] and electrostatic force are also used for drug loading [102]. The excellent drug loading capacity, chemical and mechanical stability and biocompatibility of GO have made it famous in drug delivery research areas. However, Zhu et al. [103] have found that GO could promote cancer metastasis via TGF-β signaling-dependent epithelial-mesenchymal transition, which was to alert that inadvertent or unintended effects must be concerned while designing drug delivery systems.
4.5.2. Gold nanoparticlesGold nanoparticles are famous for their excellent photothermal efficacy and surface modification ability [104]. Nam et al. [105] have synthesized pH-responsive smart gold nanoparticles for photothermal cancer therapy, the smart gold nanoparticles were pH-inducible, which could aggregate in acidic environment. The unique pH-induced behavior was utilized for tumor aggregation, later applied NIR to realize photothermal therapy precisely.
4.5.3. Black phosphorusBlack phosphorus is a stable allotrope of phosphorus elements, which shows excellent drug loading capacity and high photothermal conversion efficiency, has attracted numerous attentions in drug delivery and photothermal therapy researching in recent several years [2, 106, 107]. Black phosphorus nanosheets and black phosphorus quantum dots are most commonly used in drug delivery [2, 108, 109]. The black phosphorus nanosheet shows excellent drug loading capacity which holds 950% DOX in weight on the sheet surface, owing to the two-dimensional structure of black phosphorus nanosheets have provided extending surface area for drug loading [110]. The drug loading is realized through π-π stacking or electrostatic interaction. The surface of black phosphorus is negative charged in physiological pH, the electrostatic interaction between drug and black phosphorus is changed when it is protonated in acidic pH, as a result of inducing drug release [111].
4.5.4. Mesoporous silicaMesoporous silica nanoparticles (MSNs) are popularly served as carriers in drug delivery. On the one hand, there are numerous Si OH groups on MSNs, which can be linked with drugs via pH-sensitive bonds. On the other hand, the drug loaded MSNs can be coated via pH-sensitive material that is serving as gatekeeper. Cheng et al. [112] have fabricated a kind of novel DOX loaded mesoporous silica nanoparticles (MSN-DOX@PDA–PEG–FA), the polydopamine (PDA) was pH-sensitive material and was coated on the surface of MSN, which blocked the pores and served as a gatekeeper for the DOX releasing from MSN. The releasing progress was, while the nanoparticles encountering tumor acid environment, the loaded DOX was released rapidly because of the breakage of the PDA films. As a result, the PDA coated nanoparticles showed enhanced tumor killing effect and had better biocompatibility.
5. Other types of pH-sensitive materials 5.1. pH-Sensitive lipidsLipids are able to form liposomes that are similar to bi-layer structure of cellular membrane, the liposomes have been used for drug delivery for long time and many of them had been approved by FDA. pH-Sensitive liposomes are composed of pH-sensitive lipids containing ionizable groups, which undergo protonation in acidic environment and occur property changing so as to trigger drug releasing [113]. The most widely used pH-sensitive lipids are dioleylphosphatidylamine (DOPE) [114], polyoleoylphosphatidylethanolamine (POPE) [115], and dioleoylphosphatidylcholine (DSPC) [116].
5.2. pH-Sensitive peptidespH-Sensitive peptides are mainly including pH-sensitive cell penetrating peptides (CPP), pH-sensitive fusogenic peptides, and amphiphilic polypeptide-based supramolecular, which are capable for pH-sensitive drug delivery.
CPPs are regarded as potential carriers for intracellular drug delivery, however, lacking tumor selectivity, CPPs are also toxic to normal cells. Modifying CPPs to pH-sensitive CPPs can solve the problem [117]. Ouahab et al. [118] have prepared a pH-sensitive charge-reversal CPPs to conjugate micelle, the peptide was composed of decapeptide arginine-glycine (RG)5 and decapeptide histidine-glutamic acid (HE)5. The imidazole groups on histidine was neutral at physiological pH environment, but it was protonated at acidic pH, which changed the electrostatic interaction as a result of action sites (RG)5 exposing to exert cell penetrating ability.
pH-Sensitive fusogenic peptides are capable for changing conformation under acid environment to fuse cellular membrane, GALA [119], KALA [120, 121] and EALA [122] are most commonly investigated fusogenic peptides. Fusogenic peptides modified nanoparticles are rapidly eliminated via RES, this limitation is overcoming through PEGylation [123]. Such fusogenic peptides are especially suitable for gene delivery. Harashima et al. have prepared GALA peptide containing nanoparticle for robust gene silencing to treat metastatic lung cancer, the nanoparticle showed 40-fold more efficient than a previously developed systems [124].
Some of peptides sequences owning pH-responsibility, especially for ionizable groups containing sequences, are designed as amphiphilic supramolecular through incorporating with other structures [126]. The amphiphilic polypeptide-based supramolecular can self-assembly to nanostructure capable for on-demand drug loading and releasing. An oligo-histidine His6 sequence is an example of such pH-responsible sequences. The histidine residues are protonated greatly in acid environment (below pH 6.5), which lead to selfassembly structure disruption by electrostatic repulsion effect [126]. Wang et al. [125] have fabricated pH-sensitive nanoparticles that were self-assembled from His6 based supramolecular for homing theranostics. As shown in Fig.7, the self-assembled nanoparticles converted to nanofibers through structural transformation while transferring to acidic environment. The nanofibers were capable for capturing intravenously injected small molecular drugs, which led to drugs passive accumulation in tumor tissues, such process was a kind of homing theranostics.

|
Download:
|
| Fig. 7. An example for pH-sensitive peptide (His6) based supramolecular self-assembly nanoparticles as homing theranostics. Copied with permission [125]. Copyright 2016, Wiley. | |
6. Conclusion and perspective
Cancer remains hard to eradicate due to cancer cells appear high similarity with normal cells. The cancer cells which mutated from normal cells hold eukaryotic structure and similar immunogen property, which leads to lack of selectivity of chemotherapy. It is important to solve the problems through utilizing the differences between cancer cells and normal cells. The most popularly investigated differences are pH, redox environment, enzymes and receptors expression. These differences are used to fabricate drug delivery systems, which are designed precisely and can be triggered by tumor signals, to realize selective delivery of drugs into tumor tissues to exert better therapeutic effect. Among kinds of drug delivery systems, pH-sensitive drug delivery systems have exhibited good therapeutic effect innumerous researches, which have shown higher toxicity to cancer cells in vitro and enhanced tumor selectivity in vivo. This review mainly focuses on tumoral lower pH environment and introduces the pH-sensitive materials and their main drug release mechanism. pH-Sensitive drug delivery mechanisms mainly include pH-sensitive bond cleavage and protonation mechanism, they are theoretical basis for designing precise drug delivery systems. pH-Sensitive materials like ionizable polymers and copolymers, linker-based polymers, calcium nanoparticles, metal oxides, pH-sensitive peptides and lipids are commonly used for pH-sensitive drug delivery to enhance the anticancer therapeutic efficacy while decreasing toxicity adverse effects. pH-Sensitive drug delivery systems have propelled steps on overring the limitation of traditional chemotherapy, however, they are still facing some problems: i) long-term toxicity of the nanoparticles should be verified to ensure the systems are safe; ii) how to realize zero-release in blood circulation whereas full-release in tumor tissue is still a challenge; iii) penetration ability of the pH-sensitive drug delivery systems is not enough, which needs to be enhanced to achieve tumor deeper sites to exert better tumor killing effect.
To date, attention has been paid to combination therapy, which has been proved to be more efficacy in cancer therapy. Various novel materials that are not only capable for pH-sensitivity but also excellent in other properties, such as photo-sensitivity, magnetic resonance, or redox responding ability, are employed to realize the combination therapy [127-129]. Owing to combination therapy possesses applications in multi-drug resistance and exhibits enhanced tumor selectivity and reduced systemic toxicity, it shows promising future in cancer therapy.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by grants from the National Natural Science Foundation of China (Nos. 31922042 and 81771966) and Science, Technology & Innovation Commission of Shenzhen Municipality (No. JCYJ20160531195129079).
| [1] |
F. Bray, J. Ferlay, I. Soerjomataram, et al., CA-Cancer J. Clin. 68 (2018) 394-424. DOI:10.3322/caac.21492 |
| [2] |
M. M. Luo, T. J. Fan, Y. Zhou, et al., Adv. Funct. Mater. 29 (2019) 1808306. DOI:10.1002/adfm.201808306 |
| [3] |
C. Li, J. C. Wang, Y. G. Wang, et al., Acta Pharm. Sin. B 9 (2019) 1145-1162. DOI:10.1016/j.apsb.2019.08.003 |
| [4] |
D. B. Longley, D. P. Harkin, P. G. Johnston, Nat. Rev. Cancer 3 (2003) 330-338. DOI:10.1038/nrc1074 |
| [5] |
EarlyBreast Cancer Trialists' Collaborative Group, Lancet (London, England) 352 (1998) 930-942. DOI:10.1016/S0140-6736(98)03301-7 |
| [6] |
Y. K. Zhang, C. L. Dai, C. G. Yuan, et al., Acta Pharm. Sin. B 7 (2017) 564-570. DOI:10.1016/j.apsb.2017.04.001 |
| [7] |
H. Liu, S. Mi, Z. Li, et al., Acta Pharm. Sin. B 3 (2013) 226-233. DOI:10.1016/j.apsb.2013.05.004 |
| [8] |
Q. Xia, Y. T. Zhang, Z. Li, et al., Acta Pharm. Sin. B 9 (2019) 675-689. DOI:10.1016/j.apsb.2019.01.011 |
| [9] |
K. Park, J. Control. Release 190 (2014) 3-8. DOI:10.1016/j.jconrel.2014.03.054 |
| [10] |
S. Mura, J. Nicolas, P. Couvreur, Nat. Mater. 12 (2013) 991-1003. DOI:10.1038/nmat3776 |
| [11] |
X. Wang, X. Wang, S. Jin, et al., Chem. Rev. 119 (2019) 1138-1192. DOI:10.1021/acs.chemrev.8b00209 |
| [12] |
A. E. Felber, M. H. Dufresne, J. C. Leroux, Adv. Drug Deliv. Rev. 64 (2012) 979-992. DOI:10.1016/j.addr.2011.09.006 |
| [13] |
J. Liu, Y. R. Huang, A. Kumar, et al., Biotechnol. Adv. 32 (2014) 693-710. DOI:10.1016/j.biotechadv.2013.11.009 |
| [14] |
M. Kanamala, W. R. Wilson, M. M. Yang, et al., Biomaterials 85 (2016) 152-167. DOI:10.1016/j.biomaterials.2016.01.061 |
| [15] |
K. M. Huh, H. C. Kang, Y. J. Lee, et al., Macromol. Res. 20 (2012) 224-233. DOI:10.1007/s13233-012-0059-5 |
| [16] |
M. X. Wu, Y. W. Yang, Adv. Mater. 29 (2017) 1606134. DOI:10.1002/adma.201606134 |
| [17] |
Z. L. Dong, L. Z. Feng, W. W. Zhu, et al., Biomaterials 110 (2016) 60-70. DOI:10.1016/j.biomaterials.2016.09.025 |
| [18] |
J. Shin, P. Shum, D. H. Thompson, J. Control. Release 91 (2003) 187-200. DOI:10.1016/S0168-3659(03)00232-3 |
| [19] |
H. K. Kim, J. Van den Bossche, S. H. Hyun, et al., Bioconjugate Chem. 23 (2012) 2071-2077. DOI:10.1021/bc300266y |
| [20] |
H. Z. Deng, J. J. Liu, X. F. Zhao, et al., Biomacromolecules 15 (2014) 4281-4292. DOI:10.1021/bm501290t |
| [21] |
S. J. Zhu, M. H. Hong, G. T. Tang, et al., Biomaterials 31 (2010) 1360-1371. DOI:10.1016/j.biomaterials.2009.10.044 |
| [22] |
Z. B. Gao, L. N. Zhang, Y. J. Sun, J. Control. Release 162 (2012) 45-55. DOI:10.1016/j.jconrel.2012.05.051 |
| [23] |
C. X. Ding, J. X. Gu, X. Z. Qu, et al., Bioconjugate Chem. 20 (2009) 1163-1170. DOI:10.1021/bc800563g |
| [24] |
I. Szabo, M. Manea, E. Orban, et al., Bioconjugate Chem. 20 (2009) 656-665. DOI:10.1021/bc800542u |
| [25] |
Y. Jin, L. Song, Y. Su, et al., Biomacromolecules 12 (2011) 3460-3468. DOI:10.1021/bm200956u |
| [26] |
Z. Zhou, L. Li, Y. Yang, et al., Biomaterials 35 (2014) 6622-6635. DOI:10.1016/j.biomaterials.2014.04.059 |
| [27] |
Y. J. Pu, S. Chang, H. Yuan, et al., Biomaterials 34 (2013) 3658-3666. DOI:10.1016/j.biomaterials.2013.01.082 |
| [28] |
Z. H. Huang, X. Guo, W. J. Li, et al., J. Am. Chem. Soc. 128 (2006) 60-61. DOI:10.1021/ja057024w |
| [29] |
J. H. Shin, P. Shum, J. Grey, et al., Mol. Pharm. 9 (2012) 3266-3276. DOI:10.1021/mp300326z |
| [30] |
Y. Matsumura, Jpn. J. Clin. Oncol. 44 (2014) 515-552. DOI:10.1093/jjco/hyu046 |
| [31] |
X. W. Zeng, G. Liu, W. Tao, et al., Adv. Funct. Mater. 27 (2017) 1605985. DOI:10.1002/adfm.201605985 |
| [32] |
J. Tan, H. Li, X. X. Hu, et al., Chem 5 (2019) 1775-1792. DOI:10.1016/j.chempr.2019.05.024 |
| [33] |
V. M. Rusu, C. H. Ng, M. Wilke, et al., Biomaterials 26 (2005) 5414-5426. DOI:10.1016/j.biomaterials.2005.01.051 |
| [34] |
H. Cabral, Y. Matsumoto, K. Mizuno, et al., Nat. Nanotechnol. 6 (2011) 815-823. |
| [35] |
X. L. Chen, L. S. Liu, C. Jiang, Acta Pharm. Sin. B 6 (2016) 261-267. DOI:10.1016/j.apsb.2016.05.011 |
| [36] |
M. Zhang, X. Chen, C. Li, et al., J. Control. Release 319 (2019) 46-62. |
| [37] |
M. A. Aghdam, R. Bagheri, J. Mosafer, et al., J. Control. Release 315 (2019) 1-22. DOI:10.1016/j.jconrel.2019.09.018 |
| [38] |
J. Wen, K. Yang, F. Y. Liu, et al., Chem. Soc. Rev. 46 (2017) 6024-6045. DOI:10.1039/C7CS00219J |
| [39] |
S. Bazban-Shotorbani, M. M. Hasani-Sadrabadi, A. Karkhaneh, et al., J. Control. Release 253 (2017) 46-63. DOI:10.1016/j.jconrel.2017.02.021 |
| [40] |
L. P. Zhu, P. P. Smith, S. G. Boyes, J. Polym. Sci. Pt. B-Polym. Phys. 51 (2013) 1062-1067. DOI:10.1002/polb.23302 |
| [41] |
W. Cheng, X. W. Zeng, H. Z. Chen, et al., ACS Nano 13 (2019) 8537-8565. DOI:10.1021/acsnano.9b04436 |
| [42] |
J. F. Mano, Adv. Eng. Mater. 10 (2008) 515-527. DOI:10.1002/adem.200700355 |
| [43] |
M. D. Kurkuri, T. M. Aminabhavi, J. Control. Release 96 (2004) 9-20. DOI:10.1016/j.jconrel.003.12.025 |
| [44] |
S. J. Grainger, M. E. H. , El-sayed, Stimuli-sensitive particles for drug delivery, World Scientific Publishing Co. Pte. Ltd., Singapore (2010). |
| [45] |
S. Bersani, M. Vila-Caballer, C. Brazzale, et al., Eur. J. Pharm. Biopharm. 88 (2014) 670-682. DOI:10.1016/j.ejpb.2014.08.005 |
| [46] |
J. Zheng, X. J. Tian, Y. F. Sun, et al., Int. J. Pharm. 450 (2013) 296-303. DOI:10.1016/j.ijpharm.2013.04.014 |
| [47] |
K. Y. Lee, D. J. Mooney, Prog. Polym. Sci. 37 (2012) 106-126. DOI:10.1016/j.progpolymsci.2011.06.003 |
| [48] |
C. K. Choudhury, S. Roy, Soft Matter 9 (2013) 2269-2281. DOI:10.1039/c2sm26290h |
| [49] |
M. Bilal, T. Rasheed, Y. P. Zhao, et al., Int. J. Biol. Macromol. 124 (2019) 742-749. DOI:10.1016/j.ijbiomac.2018.11.220 |
| [50] |
F. S. Majedi, M. M. Hasani-Sadrabadi, J. J. VanDersarl, et al., Adv. Funct. Mater. 24 (2014) 432-441. DOI:10.1002/adfm.201301628 |
| [51] |
L. Wang, G. H. Liu, X. R. Wang, et al., Macromolecules 48 (2015) 7262-7272. DOI:10.1021/acs.macromol.5b01709 |
| [52] |
A. D. Wang, W. Y. Shi, J. B. Huang, et al., Soft Matter 12 (2016) 337-357. DOI:10.1039/C5SM02397A |
| [53] |
Y. Wang, Z. Luan, C. Zhao, et al., Eur. J. Pharm. Sci. 142 (2020) 105136. DOI:10.1016/j.ejps.2019.105136 |
| [54] |
G. C. Yu, R. Zhao, D. Wu, et al., Polym. Chem. 7 (2016) 6178-6188. DOI:10.1039/C6PY01402J |
| [55] |
Y. Y. Zhang, C. Teh, M. H. Li, et al., Chem. Mat. 28 (2016) 7039-7050. DOI:10.1021/acs.chemmater.6b02896 |
| [56] |
X. X. Huang, W. B. Liao, Z. H. Xie, et al., Mater. Sci. Eng. C 90 (2018) 27-37. DOI:10.1016/j.msec.2018.04.036 |
| [57] |
H. Furukawa, K. E. Cordova, M. O'Keeffe, et al., Science 341 (2013) 974-987. |
| [58] |
B. F. Hoskins, R. Robson, J. Am. Chem. Soc. 111 (1989) 5962-5964. DOI:10.1021/ja00197a079 |
| [59] |
H. Q. Zheng, Y. N. Zhang, L. F. Liu, et al., J. Am. Chem. Soc. 138 (2016) 962-968. DOI:10.1021/jacs.5b11720 |
| [60] |
J. Zhuang, C. H. Kuo, L. Y. Chou, et al., ACS Nano 8 (2014) 2812-2819. DOI:10.1021/nn406590q |
| [61] |
C. Y. Sun, C. Qin, X. L. Wang, et al., Dalton Trans. 41 (2012) 6906-6909. DOI:10.1039/c2dt30357d |
| [62] |
K. S. Park, Z. Ni, A. P. Cote, et al., Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 10186-10191. DOI:10.1073/pnas.0602439103 |
| [63] |
M. Kandiah, M. H. Nilsen, S. Usseglio, et al., Chem. Mat. 22 (2010) 6632-6640. DOI:10.1021/cm102601v |
| [64] |
M. Latroche, S. Surble, C. Serre, et al., Angew. Chem. Int. Ed. 45 (2006) 8227-8231. DOI:10.1002/anie.200600105 |
| [65] |
D. D. Wang, J. J. Zhou, R. H. Chen, et al., Biomaterials 100 (2016) 27-40. DOI:10.1016/j.biomaterials.2016.05.027 |
| [66] |
H. Lee, S. M. Dellatore, W. M. Miller, et al., Science 318 (2007) 426-430. DOI:10.1126/science.1147241 |
| [67] |
Q. Wu, M. Niu, X. W. Chen, et al., Biomaterials 162 (2018) 132-143. DOI:10.1016/j.biomaterials.2018.02.022 |
| [68] |
S. M. Dizaj, M. Barzegar-Jalali, M. H. Zarrintan, et al., Expert Opin. Drug Deliv. 12 (2015) 1649-1660. DOI:10.1517/17425247.2015.1049530 |
| [69] |
M. Motskin, D. M. Wright, K. Muller, et al., Biomaterials 30 (2009) 3307-3317. DOI:10.1016/j.biomaterials.2009.02.044 |
| [70] |
M. Li, S. Mann, Adv. Funct. Mater. 12 (2002) 773-779. DOI:10.1002/adfm.200290006 |
| [71] |
Z. S. Hu, Y. L. Deng, Q. H. Sun, Colloid J. 66 (2004) 745-750. DOI:10.1007/s10595-005-0017-4 |
| [72] |
Y. Ueno, H. Futagawa, Y. Takagi, et al., J. Control. Release 103 (2005) 93-98. DOI:10.1016/j.jconrel.2004.11.015 |
| [73] |
Y. Zhang, P. Ma, Y. Wang, et al., World J. Nano Sci. Eng. 02 (2012) 25-31. DOI:10.4236/wjnse.2012.21005 |
| [74] |
A. Som, R. Raliya, L. M. Tian, et al., Nanoscale 8 (2016) 12639-12647. DOI:10.1039/C5NR06162H |
| [75] |
C. S. Zhou, T. Chen, C. C. Wu, et al., Chem. -Asian J. 10 (2015) 166-171. DOI:10.1002/asia.201403115 |
| [76] |
M. Bohner, Eur. Cells Mater. 20 (2010) 1-12. DOI:10.22203/eCM.v020a01 |
| [77] |
W. Habraken, P. Habibovic, M. Epple, et al., Mater. Today 19 (2016) 69-87. DOI:10.1016/j.mattod.2015.10.008 |
| [78] |
S. V. Dorozhkin, Acta Biomater. 6 (2010) 715-734. DOI:10.1016/j.actbio.2009.10.031 |
| [79] |
C. Combes, C. Rey, Acta Biomater. 6 (2010) 3362-3378. DOI:10.1016/j.actbio.2010.02.017 |
| [80] |
H. R. R. Ramay, M. Zhang, Biomaterials 25 (2004) 5171-5180. DOI:10.1016/j.biomaterials.2003.12.023 |
| [81] |
J. Li, Y. C. Chen, Y. C. Tseng, et al., J. Control. Release 142 (2010) 416-421. DOI:10.1016/j.jconrel.2009.11.008 |
| [82] |
L. H. Fu, R. Y. -Hu, C. Qi, et al., ACS Nano 13 (2019) 13985-13994. DOI:10.1021/acsnano.9b05836 |
| [83] |
Y. F. Rao, W. Chen, X. G. Liang, et al., Small 11 (2015) 239-247. DOI:10.1002/smll.201400775 |
| [84] |
M. Lu, M. H. Cohen, D. Rieves, et al., Am. J. Hematol. 85 (2010) 315-319. |
| [85] |
S. Zanganeh, G. Hutter, R. Spitler, et al., Nat. Nanotechnol. 11 (2016) 986-994. |
| [86] |
S. Xu, J. Liu, D. Li, et al., Biomaterials 35 (2014) 1676-1685. DOI:10.1016/j.biomaterials.2013.10.081 |
| [87] |
M. Y. Wu, Q. S. Meng, Y. Chen, et al., Adv. Funct. Mater. 24 (2014) 4273-4283. DOI:10.1002/adfm.201400256 |
| [88] |
J. X. Lu, J. H. Sun, F. Y. Li, et al., J. Am. Chem. Soc. 140 (2018) 10071-10074. DOI:10.1021/jacs.8b04169 |
| [89] |
T. Kim, E. J. Cho, Y. Chae, et al., Angew. Chem. Int. Ed. 50 (2011) 10589-10593. DOI:10.1002/anie.201103108 |
| [90] |
Y. Chen, D. L. Ye, M. Y. Wu, et al., Adv. Mater. 26 (2014) 7019-7026. DOI:10.1002/adma.201402572 |
| [91] |
J. J. Liu, Q. Chen, W. W. Zhu, et al., Adv. Funct. Mater. 27 (2017). |
| [92] |
B. Y. W. Hsu, M. Ng, A. Tan, et al., Adv. Healthc. Mater. 5 (2016) 721-729. DOI:10.1002/adhm.201500908 |
| [93] |
A. Sasidharan, P. Chandran, D. Menon, et al., Nanoscale 3 (2011) 3657-3669. DOI:10.1039/c1nr10272a |
| [94] |
M. J. Akhtar, M. Ahamed, S. Kumar, et al., Int. J. Nanomed. 7 (2012) 845-857. |
| [95] |
X. L. Cai, Y. N. Luo, H. Y. Yan, et al., ACS Appl. Mater. Interfaces 9 (2017) 5739-5747. DOI:10.1021/acsami.6b13776 |
| [96] |
J. Wang, J. S. Lee, D. Kirn, et al., ACS Appl. Mater. Interfaces 9 (2017) 39971-39984. DOI:10.1021/acsami.7b11219 |
| [97] |
K. S. Novoselov, A. K. Geim, S. V. Morozov, et al., Science 306 (2004) 666-669. DOI:10.1126/science.1102896 |
| [98] |
D. R. Dreyer, S. Park, C. W. Bielawski, et al., Chem. Soc. Rev. 39 (2010) 228-240. DOI:10.1039/B917103G |
| [99] |
J. Q. Liu, L. Tao, W. R. Yang, et al., Langmuir 26 (2010) 10068-10075. DOI:10.1021/la1001978 |
| [100] |
D. Depan, J. Shah, R. D. K. Misra, Mater. Sci. Eng. C 31 (2011) 1305-1312. DOI:10.1016/j.msec.2011.04.010 |
| [101] |
J. F. Shen, M. Shi, N. Li, et al., Nano Res. 3 (2010) 339-349. DOI:10.1007/s12274-010-1037-x |
| [102] |
J. Q. Liu, L. Cui, D. Losic, Acta Biomater. 9 (2013) 9243-9257. DOI:10.1016/j.actbio.2013.08.016 |
| [103] |
J. Zhu, B. Li, M. Xu, et al., ACS Nano 14 (2020) 818-827. DOI:10.1021/acsnano.9b07891 |
| [104] |
X. J. Cheng, R. Sun, L. Yin, et al., Adv. Mater. 29 (2017) 1604894. DOI:10.1002/adma.201604894 |
| [105] |
J. Nam, N. Won, H. Jin, et al., J. Am. Chem. Soc. 131 (2009) 13639-13645. DOI:10.1021/ja902062j |
| [106] |
X. Liang, X. Y. Ye, C. Wang, et al., J. Control. Release 296 (2019) 150-161. DOI:10.1016/j.jconrel.2019.01.027 |
| [107] |
Y. Zhang, Y. Zheng, K. Rui, et al., Small 13 (2017) 1700661. DOI:10.1002/smll.201700661 |
| [108] |
W. Tao, X. B. Zhu, X. H. Yu, et al., Adv. Mater. 29 (2017) 1603276. DOI:10.1002/adma.201603276 |
| [109] |
X. W. Zeng, M. M. Luo, G. Liu, et al., Adv. Sci. 5 (2018) 1603276. |
| [110] |
W. S. Chen, J. Ouyang, H. Liu, et al., Adv. Mater. 29 (2017) 1603864. DOI:10.1002/adma.201603864 |
| [111] |
F. Zhang, F. F. Peng, L. Qin, et al., Colloid Surf. B 180 (2019) 353-361. DOI:10.1016/j.colsurfb.2019.04.021 |
| [112] |
W. Cheng, J. P. Nie, L. Xu, et al., ACS Appl. Mater. Interfaces 9 (2017) 18462-18473. DOI:10.1021/acsami.7b02457 |
| [113] |
S. R. Paliwal, R. Paliwal, S. P. Vyas, Drug Deliv. 22 (2015) 231-242. DOI:10.3109/10717544.2014.882469 |
| [114] |
M. Z. Lai, N. Duzgunes, F. C. Szoka, Biochemistry 24 (1985) 1646-1653. |
| [115] |
E. Torres, F. Mainini, R. Napolitano, et al., J. Control. Release 154 (2011) 196-202. DOI:10.1016/j.jconrel.2011.05.017 |
| [116] |
M. S. Webb, J. J. Wheeler, M. B. Bally, et al., Biochim. Biophys. Acta 1238 (1995) 147-155. DOI:10.1016/0005-2736(95)00121-I |
| [117] |
L. K. Fei, L. P. Yap, P. S. Conti, et al., Biomaterials 35 (2014) 4082-4087. DOI:10.1016/j.biomaterials.2014.01.047 |
| [118] |
A. Ouahab, N. Cheraga, V. Onoja, et al., Int. J. Pharm. 466 (2014) 233-245. DOI:10.1016/j.ijpharm.2014.03.009 |
| [119] |
N. K. Subbarao, R. A. Parente, F. C. Szoka Jr. , et al., Biochemistry 26 (1987) 2964-2972. |
| [120] |
T. B. Wyman, F. Nicol, O. Zelphati, et al., Biochemistry 36 (1997) 3008-3017. |
| [121] |
X. D. Guo, N. Wiradharma, S. Q. Liu, et al., Biomaterials 33 (2012) 6284-6291. DOI:10.1016/j.biomaterials.2012.05.033 |
| [122] |
C. Dalgicdir, C. Globisch, M. Sayar, et al., Eur. Phys. J. -Spec. Top. 225 (2016) 1463-1481. DOI:10.1140/epjst/e2016-60147-8 |
| [123] |
T. Nakamura, H. Akita, Y. Yamada, et al., Accounts Chem. Res. 45 (2012) 1113-1121. DOI:10.1021/ar200254s |
| [124] |
M. M. Abd Elwakil, I. A. Khalil, Y. H. A. Elewa, et al., Adv. Funct. Mater. 29 (2019) 1807677. DOI:10.1002/adfm.201807677 |
| [125] |
P. P. Yang, Q. Luo, G. B. Qi, et al., Adv. Mater. 29 (2017) 1605869. DOI:10.1002/adma.201605869 |
| [126] |
T. J. Moyer, J. A. Finbloom, F. Chen, et al., J. Am. Chem. Soc. 136 (2014) 14746-14752. DOI:10.1021/ja5042429 |
| [127] |
J. N. Hu, Y. Xu, Y. Zhang, Chin. Chem. Lett. 30 (2019) 2039-2042. DOI:10.1016/j.cclet.2019.05.017 |
| [128] |
W. X. Lv, X. Wang, J. H. Wu, et al., Chin. Chem. Lett. 30 (2019) 1635-1638. DOI:10.1016/j.cclet.2019.06.029 |
| [129] |
X. M. Li, Y. H. Zhang, Z. Q. Ma, et al., Chin. Chem. Lett. 30 (2019) 489-493. DOI:10.1016/j.cclet.2018.03.019 |
 2020, Vol. 31
2020, Vol. 31