b State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Coordinatively unsaturated zero-valent transition-metal species are putative intermediates in many transition-metal-catalyzed reactions and have thus intrigued great synthetic interests on analogous isolable complexes [1-5]. So far, the most well-studied coordinatively unsaturated zero-valent metal complexes are the three- and two-coordinate group 10 metal (Ni, Pd, Pt) complexes [6-8], and coordinatively unsaturated zero-valent metal complexes of the other transition-metals are mainly known for the ones with coordination numbers of five and four [9-12]. As for the three- and two-coordinate zero-valent metal complexes of group 4–9 metals, the two-coordinate complexes with cyclic (alkyl)(amino)carbene (cAAC) ligation M(cAAC)2 (M = Co, Fe, Mn) [13], the inorganic Grignard reagent-type complex [(Mesnacnac)-MgMn(N(C6H2-2, 6-(CHPh2)2-4-Pri)(SiPr3i))] [14], and the threecoordinate complexes with persistent carbene and vinylsilane ligation (L)M(η2-CH2CHSiR3)2 (L = N-heterocyclic carbene (NHC), cAAC; M = Co, Fe, Mn) [15-19] are among the rarely known examples. In these cases, the π-accepting nature of the ligand environment plays a key role for the stabilization of the low-coordinate low-valent metal complexes. The success in the preparation of three-coordinate formal cobalt(0), iron(0), and manganese(0) complexes with the combined ligand set of persistent carbenes and vinylsilanes prompted investigations on the applicability of other alkene ligands in combination with NHCs in stabilizing analogous low-coordinate metal complexes. Herein, we wish to communicate the finding that the readily available alkene, styrene, in combination with NHC fulfills this task.
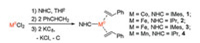
The synthesis of the three-coordinate formal zero-valent metal styrene complexes can be achieved by the one-pot reaction protocol similar to that used for the preparation of (NHC)M(η2- CH2CHSiMe3)2. Stirring the mixture of MCl2 (M = Co, Fe, Mn) with 1 equiv. of NHC in THF at room temperature for hours led to the formation of clear solutions, to which styrene (2 equiv.) and potassium graphite (2 equiv.) were added subsequently. The reaction mixtures were then kept stirring at room temperature for hours. Further workup on the resultant mixtures led to the isolation of the styrene complexes [(IMes)Co(η2-CH2CHPh)2] (1, IMes = 1, 3-bis(2', 4', 6'-trimethylphenyl)imidazol-2-ylidene), [(IPr)-Fe(η2-CH2CHPh)2] (2, IPr = 1, 3-bis(2', 6'-diisopropylphenyl)imidazol-2-ylidene), [(IMes)Fe(η2-CH2CHPh)2] (3), and [(IPr)Mn(η2-CH2CHPh)2] (4) in 40%–62% yields (Scheme 1). These isolated yields are comparable to those of the corresponding vinylsilanes complexes [15-19].
|
Download:
|
| Scheme 1. Synthetic route to [(NHC)M(η2-CH2CHPh)2]. | |
Complexes 1-4 are air- and moisture-sensitive, quite soluble in THF, and slightly soluble in diethyl ether and toluene. Their absorption spectra measured in THF exhibit two intensive bands in the UV–vis region with the absorption maxima around 300 and 330 nm. The large absorption coefficients of these bands (more than 1000 dm-3 cm-1) indicate their charge-transfer nature. Being similar to the vinylsilane complexes (NHC)M(η2:η2-dvtms) and (NHC)M(η2-CH2CHSiMe3)2 (M = Co, Fe, Mn) [15-19], the styrene complexes 1-4 also show two characteristic absorption bands in region of 600-900 nm (670 and 830 nm for 1, 640 and 780 nm for 2 and 3, and 650 and 770 nm for 4). Complexes 1-4 are paramagnetic. Their solution magnetic moments measured by Evans' method are comparable to those of the corresponding vinylsilane complexes (NHC)M(η2:η2-dvtms) (M = Co, Fe, Mn) [15-19].
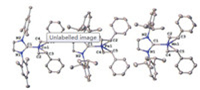
Single-crystal X-ray diffraction studies unambiguously confirmed the three-coordinate nature of 1-4 (CCDC: 1957350- 1957353). Fig. 1 shows the structures of 1, 2, and 4. Their key interatomic distances and angles are compiled in Table S2 (Supporting information). The structure of 3 can be find in Fig. S1 (Supporting information). In these formal zero-valent metal complexes, the styrene ligands are coordinating to the metal centers via their vinyl moiety in an η2-fashion, and the vinyl groups are nearly coplanar with their phenyl rings. Being typical of threecoordinate formal zero-valent metal complexes with alkene and NHC ligation, the Calkene-Calkene bonds in 1-4 with the distances around 1.42 Å locate between typical C--C single bond and C=C double bond, and their M-Ccarbene bonds are on the short end of the M-Ccarbene bonds of three-coordinate metal-NHC complexes [20, 21]. Probably due to the different steric nature of Ph versus SiMe3, the dihedral angle between the imidazole plane of the NHC ligands and the idealized plane defined by the metal center and the four Calkene atoms in 1-4 are slightly smaller than those of their corresponding vinylsilane complexes (Table S2). Despite this difference, the M-Ccarbene, M-Calkene, and Calkene-Calkene distances in 1-4 are comparable to corresponding bond distances of the formal zero-valent metal vinylsilane complexes [15-19].
|
Download:
|
| Fig. 1. Molecular structures of [(IMes)Co(η2-CH2CHPh)2] (1, left) [(IPr)Fe(η2- CH2CHPh)2] (2, middle), and [(IPr)Mn(η2-CH2CHPh)2] (4, right), showing 30% probability ellipsoids and the partial atom numbering scheme. Hydrogen atoms are omitted for clarity. For simplicity, only one of the three crystallographically independent molecules in the unit cells of 2 and 4 are shown. | |
Calculations on [(IPr)Fe(η2-CH2CHPh)2] (2) at the B3LYP/TZVP level of theory [22-24] indicate that the complex with an S = 1 ground spin-state has an electronic configuration of (dxy+πC=C*)2(dx2-y2+π'C=C*)2(dz2)2(dxz)1(dyz)1 and that the π-backdonation in 2 when gauged by the contribution of the 3d orbitals (69% and 64% Fe 3d in UNOs 172 and 173, Fig. S8 in Supporting information) is comparable to that in the three-coordinate iron(0) vinylsilane complex [(IPr)Fe(η2-CH2CHSiMe3)2] (61% and 72% Fe 3d in its π-bonding orbitals) [19]. Being consistent with their comparable π-accepting nature, the addition of 2 equiv. of CH2CHPh into the C6D6 solution of [(IPr)Fe(η2-CH2CHSiMe3)2] gave a mixture of [(IPr)Fe(η2-CH2CHPh)2] and [(IPr)Fe(η2-CH2CHSiMe3)2].
One intriguing reactivity of three-coordinate formal cobalt(0) and iron(0) vinylsilane complexes is the redox reactions with substrates [15-19]. This reactivity feature seems to be retained in the styrene complexes (NHC)M(η2-CH2CHPh)2 as the preliminary reactivity study indicated that the reaction of [(IMes)Fe(η2-CH2CHPh)2] with 2 equiv. of the organic azide DippN3 (Dipp: 2, 6-diisopropylphenyl) can produce the three-coordinate metal imido complexes [(IMes)Fe(NDipp)2] [25] and styrene in quantitative yield (Eq. 1 and Fig. S13 in Supporting information).
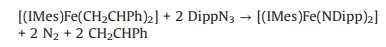
|
(1) |
In summary, we found that styrene with monodentate NHC is an effective ligand set for the stabilization of three-coordinate formal cobalt(0), iron(0), and manganese(0) complexes in the form of [(NHC)M(η2-CH2CHPh)2]. These styrene complexes have their structure and electronic features similar to the vinylsilane complexes [(NHC)M(η2-CH2CHSiMe3)2], and can also perform redox reactions with DippN3 to form three-coordinate formal M(Ⅳ) imido complexes (NHC)M(NDipp)2. The similarity hints at the synthetic utility of the NHC-metal-styrene complexes as new synthons of the mono-coordinate species (NHC)M(0). In addition, these formal zero-valent metal complexes might have potential application as new chemical vapor deposition precursors [26], and their volatility is now under investigation.
Declaration of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentsWe sincerely thank the financial support from the National Natural Science Foundation of China (Nos. 21725104, 21690062, 21432001, and 21821002), the National Key Research and Development Program (No. 2016YFA0202900), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000), and the Program of Shanghai Academic Research Leader (No. 19XD1424800).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.019.
| [1] |
D.C. Bradley, M.H. Chisholm, Acc. Chem. Res. 9 (1976) 273-280. DOI:10.1021/ar50103a005 |
| [2] |
C.C. Cummins, Prog. Inorg. Chem. 47 (1998) 685-836. |
| [3] |
P.P. Power, Chem. Rev. 112 (2012) 3482-3507. DOI:10.1021/cr2004647 |
| [4] |
P.L. Holland, Acc. Chem. Res. 41 (2008) 905-914. DOI:10.1021/ar700267b |
| [5] |
Y.C. Tsai, Coord. Chem. Rev. 256 (2012) 722-758. DOI:10.1016/j.ccr.2011.12.012 |
| [6] |
R. Ugo, Coord. Chem. Rev. 3 (1968) 319-344. DOI:10.1016/S0010-8545(00)80121-6 |
| [7] |
H.F. Klein, Angew. Chem. Int. Ed. 19 (1980) 362-375. DOI:10.1002/anie.198003621 |
| [8] |
B.R. Barnett, J.S. Figueroa, Chem. Commun. 52 (2016) 13829-13839. DOI:10.1039/C6CC07863J |
| [9] |
H. Schonberg, S. Boulmaaz, M. Worle, et al., Angew.Chem. Int. Ed. 37 (1998) 1423-1426. DOI:10.1002/(SICI)1521-3773(19980605)37:10<1423::AID-ANIE1423>3.0.CO;2-X |
| [10] |
G.W. Margulieux, N. Weidemann, D.C. Lacy, et al., J. Am. Chem. Soc. 132 (2010) 5033-5035. DOI:10.1021/ja1012382 |
| [11] |
H. Hoberg, K. Jenni, K. Angermund, C. Krüger, Angew. Chem. Int. Ed. 26 (1987) 153-155. DOI:10.1002/anie.198701531 |
| [12] |
S. Geier, R. Goddard, S. Holle, et al., Organometallics 16 (1997) 1612-1620. DOI:10.1021/om9610004 |
| [13] |
S. Roy, K.C. Mondal, H.W. Roesky, Acc. Chem. Res. 49 (2016) 357-369. DOI:10.1021/acs.accounts.5b00381 |
| [14] |
J. Hicks, C.E. Hoyer, B. Moubaraki, et al., J. Am. Chem. Soc. 136 (2014) 5283-5286. DOI:10.1021/ja5021348 |
| [15] |
H. Zhang, Z. Ouyang, Y. Liu, et al., Angew. Chem. Int. Ed. 53 (2014) 8432-8436. DOI:10.1002/anie.201404677 |
| [16] |
L. Zhang, Y. Liu, L. Deng, J. Am. Chem. Soc. 136 (2014) 15525-15528. DOI:10.1021/ja509731z |
| [17] |
J. Sun, Y. Gao, L. Deng, Inorg. Chem. 56 (2017) 10775-10784. DOI:10.1021/acs.inorgchem.7b01763 |
| [18] |
J. Cheng, Q. Chen, X. Leng, et al., Chem. 4 (2018) 2844-2860. DOI:10.1016/j.chempr.2018.09.002 |
| [19] |
J. Cheng, Q. Chen, X. Leng, S. Ye, L. Deng, Inorg. Chem. 58 (2019) 13129-13141. DOI:10.1021/acs.inorgchem.9b02009 |
| [20] |
K. Riener, S. Haslinger, A. Raba, et al., Chem. Rev. 114 (2014) 5215-5272. DOI:10.1021/cr4006439 |
| [21] |
A.A. Danopoulos, T. Simler, P. Braunstein, Chem. Rev. 119 (2019) 3730-3961. DOI:10.1021/acs.chemrev.8b00505 |
| [22] |
A.D. Becke, J. Chem. Phys. 98 (1993) 5648-5652. DOI:10.1063/1.464913 |
| [23] |
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37 (1988) 785-789. DOI:10.1103/PhysRevB.37.785 |
| [24] |
F. Neese, F. Wennmohs, A. Hansen, U. Becker, Chem. Phys. 356 (2009) 98-109. DOI:10.1016/j.chemphys.2008.10.036 |
| [25] |
L. Wang, L. Hu, H. Zhang, H. Chen, L. Deng, J. Am. Chem. Soc. 137 (2015) 14196-14207. DOI:10.1021/jacs.5b09579 |
| [26] |
K. Lubitz, V. Sharma, S. Shukla, et al., Organometallics 37 (2018) 1181-1191. DOI:10.1021/acs.organomet.8b00060 |
 2020, Vol. 31
2020, Vol. 31 

