In 1995, Lu and coworkers firstly reported a phosphinecatalyzed [3 + 2] annulation reaction to construct monocyclopentene derivatives by using 2, 3-butadienoates with electron-deficient olefins [1]. From then on, the nucleophilic phosphine-catalyzed annulation reactions have emerged as one of the most powerful tools for the construction of diverse carbocyclic and heterocyclic systems [2-15]. In the conventional phosphinecatalyzed reactions, the initial nucleophilic attack of phosphines to electron-deficient allenes, alkynes, and the modified allylic carbonates generated active zwitterionic intermediates, followed by subsequent addition to other electrophiles in a multiple step manner to assembly of versatile five-membered carbo- and heterocycles [16-33]. With the explosive development of phosphine catalyzed domino reactions, electron-deficient alkynes such as dialkyl but-2-ynedioates, alkyl propionates and benzoylacetylenes have been widely used to give active zwitterionic intermediates in many domino [3 + 2] cycloaddition reactions [33-47]. In recent years, another electron-deficient alkynes, dialkyl hex-2- en-4-ynedioates, have emerged as new reactive substrates in the phosphine-catalyzed reaction [47-52]. Dialkyl hex-2-en-4- ynedioates can be easily prepared in nearly quantitative yields from base-catalyzed dimerization of alkyl propiolates under mild reaction conditions [53-57]. Chuang and coworkers established several phosphine-catalyzed cycloaddition of dialkyl hex-2-en-4- ynedioates with various electrophiles containing C=O, C=C and C=N double bonds [58-61]. Inspired by these pioneering works and in continuation of our interest in developing new efficient synthetic protocols for the heterocyclic spiro compounds, we have also developed several domino reactions by employing nucleophilic triphenylphosphine addition to electron-deficient alkynes as key protocol for the efficient construction of diverse polycyclic and spiro compounds [62-67]. The initially formed active delocalized zwitterionic intermediates, which were generated from addition of phosphine to dialkyl hex-2-en-4-ynedioates, showed versatile reactivity of both 1, 3-dipole and 1, 5-dipole in the domino reaction depending on the chemical structures of the substrates and reaction conditions. In order to further demonstrate the potential synthetic values of the nucleophilic phosphine catalyzed annulation reaction, herein we wish to report interesting regioselectivity and diastereoselectivity of triphenylphosphine promoted domino reaction of dimethyl hex-2-en-4-ynedioate and dimethyl but-2- ynedioate with two α, α'-diactivated cyclic alkenes such as arylidene N, N'-dimethylbarbituric acids and Meldrum acids.
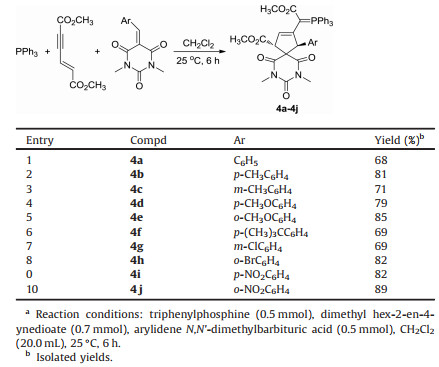
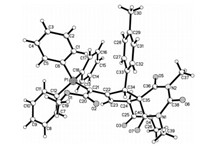
According to our previously established reaction conditions for the triphenylphosphine catalyzed domino reactions of electrondeficient alkynes [65], a mixture of triphenylphosphine 1, dimethyl hex-2-en-4-ynedioate 2 and benzylidene N, N'-dimethylbarbituric acid 3 in methylene dichloride was stirred at 25 ℃ for 6 h. After workup, the expected 6, 8, 10-trioxo-7, 9-diazaspiro[4.5]dec-1-enes 4a was obtained 68% yield. The scaffold of triphenylphosphine was retained in the final product as a triphenyl-λ5-phosphanylidene unit, which is a common phenomenon for the triphenylphosphine promoted domino reactions of electron-deficient alkynes. Then, various arylidene N, N'-dimethylbarbituric acids were employed in the reaction. The results are summarized in Table 1. All reactions proceeded smoothly to give the functionalized 6, 8, 10-trioxo-7, 9- diazaspiro[4.5]dec-1-enes 4a-4j in high yields. Although there are two chiral carbon atoms in the products 4a-4j, TLC monitoring showed that only one diastereoisomer was predominately formed in the reaction. The structures of the obtained spiro compounds 4a-4j were fully characterized by IR, HRMS, 1H, 13C and 31P NMR spectra. In the 1H NMR spectrum of the compound 4a, the three protons on the ring of cyclopentene display a singlet at δ 4.73 and two doublets at δ 4.12, δ 3.84 with coupling constant J =7.6 Hz. The two methyl groups showed two singlets at δ 3.43, δ 3.05 and two N-methyl groups showed two singlets at δ 2.84, δ 2.72. The single crystal structures of the compounds 4b (Fig. 1) and 4f (Fig. S1 in Supporting information) were successfully determined by X-ray diffraction method, crystallographic data 4b (CCDC No. 1907398), 4f (CCDC No. 1907399) have been deposited at the Cambridge Crystallographic Data base Centre. We were pleased to find that the two single crystals have same relative configuration. From the Fig. 1, it can be seen that a cyclopentene ring was formed. The 2-methoxy-2-oxo-1-(triphenyl-λ5-phosphanylidene)ethyl group connected on the cyclic C=C double bond. The aryl group and the methyl ester unit exist on trans-1, 3-positions. Therefore, we have assigned all spiro compounds 4a-4j have trans-1, 3-configuration on the basis of the spectroscopy and single crystal structures. It should be pointed out that the spiro compounds 4a-4j have the similar structural pattern to that of our previously reported reaction of triphenylphosphine, dimethyl hex-2-en-4- ynedioate and 2-arylidene-1, 3-indanediones [65].
|
|
Table 1 Synthesis of 6, 8, 10-trioxo-7, 9-diazaspiro[4.5]dec-1-enes 4a-4j.a |
|
Download:
|
| Fig. 1. Molecular structure of the compound 4b. | |
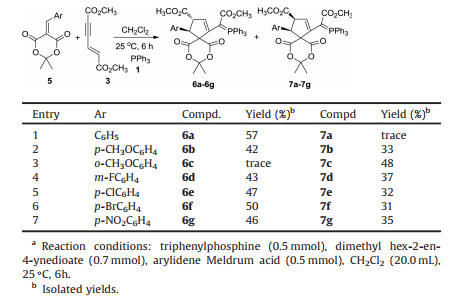
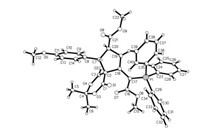
In order to demonstrate the synthetic values of this domino reaction, various arylidene Meldrum acids 5 were also tested in the three-component reaction under same reaction conditions. The results are summarized in Table 2. Although the similar spiro compounds were obtained in this reaction, the reactions with arylidene Meldrum acids gave very different regioselectivity and diastereoselectivity to that of the above mentioned reaction with arylidene N, N'-dimethylbarbituric acids. At first, different from the formation of spiro compounds with aryl group and methoxycarbonyl group at 1, 3-positions in the reaction of arylidene N, N'-dimethylbarbituric acids, the spiro compounds with aryl group and 3-methoxycarbonyl group at 1, 2-positions were obtained in the reaction containing arylidene Meldrum acids. Secondly, the reaction usually gave trans-2, 3-disubstituted 6, 10- dioxo-7, 9-dioxaspiro[4.5]dec-1-enes 6a-6g as main products and cis-2, 3-6, 10-dioxo-7, 9-dioxaspiro[4.5]dec-1-enes 7a-7g as minor products. In the reaction of benzylidene Meldrum acid, the expected cis-isomer 7a was not obtained due to too lower yield. However, only cis-isomer 7c was obtained in the reaction of o-methoxybenzylidene Meldrum acid, while the expected trans-isomer 6c was not successfully isolated. It should be pointed out that a mixture of cis/trans-isomers with similar molecular ratios were also obtained when the three-component reaction was carried out in other solvent such as dimethoxyethane, tetrahydrofuran and acetonitrile, The structures of the obtained cis/trans-isomers 6a-6g and 7a-7g were fully characterized by IR, HRMS, 1H, 13C and 31P NMR spectroscopies. The 1H NMR spectra of the cis/ trans-isomers give very different patterns of the characteristic absorptions. For example, the 1H NMR spectrum of 6b shows a singlet at δ 4.40 and a mixed peak at δ 4.12-4.03 for the three protons in cyclopentene ring. The three methoxy group give three singlets at δ 3.27, δ 2.96, δ 2.27, the one methyl groups in propylene unit shows a normal peak at δ 1.79, while the sign of another methyl group shifts to the high magnetic field at δ 0.60 due to effect of ring current of phenyl group. In the 1H NMR spectrum of 7b, the three protons on the ring of cyclopentene display a singlet at δ 4.77, and two doublets at δ 4.20, δ 3.96. The three methoxy group display three singlets at δ 2.98, δ 2.85, δ 2.25 and two methyl groups show two singlets at 1.88 and δ 1.80. The single crystal structures of the three-couples of cis/trans-isomers 6b (Fig. 2), 7b (Fig. 3), 6f, 7f, 6g and 7g (Figs. S2-S5 in Supporting information) were successfully determined by X-ray diffraction method. Crystallographic data 6b (CCDC No. 1907400), 6f (CCDC No. 1907401), 6g (CCDC No.1907402), 7b (CCDC No.1907403), 7f (CCDC No. 1907404), 7g (CCDC No. 1907405) have been deposited at the Cambridge Crystallographic Data base Centre. From the Fig. 1, it is clearly observed that the 2-aryl group and 3-methoxycarbonyl group exist in trans-configuration in spiro compound 6b. The one methyl group actually stands on the upper position of phenyl ring, which causes its absorption shifting to a high magnetic field in 1H NMR spectrum. On the other hand, the 2-aryl group and 3-methoxycarbonyl group exist on cis-configuration in spiro compound 7b. The two methyl groups do not exist on the top of phenyl ring. Thus, the two methyl groups give the normal absorption peaks in 1H NMR spectrum.
|
|
Table 2 Synthesis of cis/trans-6, 10-dioxo-7, 9-dioxaspiro[4.5]dec-1-enes.a |
|
Download:
|
| Fig. 2. Single crystal structures of the trans-isomer 6b. | |

|
Download:
|
| Fig. 3. Single crystal structure of the cis-isomer 7b. | |
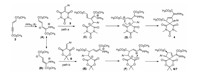
For explaining the formation of two kinds of spiro compounds in the three-component reaction, a plausible reaction mechanism is rationally proposed in Scheme 1 based on the present experiments and the previuosly reported similar reactions [62-66]. At first, addition of triphenylphosphine to C≡C triple bond of dimethyl hex-2-en-4-ynedioate gave the active dipolar intermediate A, which has a resonance hybride B via allylic immigration. Both active dipolar intermediate A and B could take part in the sequential annulation reaction. On path a, the addition of dipolar spice A to arylidene N, N'-dimethylbarbituric acid resulted in a adduct intermediate C. Then, intemediate C in turn converted to a triphenylphosphanylidene intermediate D by allylic arrangement of carbanion. Finally, the intramolecular coupling of the positive charge with the negative charge in intermediate D gave the final 6, 8, 10-trioxo-7, 9-diazaspiro[4.5]dec-1-enes 4. Because all three sequential reaction was in the retro-equilibrium reactions, it is nature to find that the stable trans-isomer was predominatedly formed as the main product in the domino reaction. On path b. the addition of active dipolar spice B to arylidene Meldrum acid resulted in a adduct intermediate E, which also converted to a triphenylphosphanylidene intermediate F and sequentially afforded the 6, 10-dioxo-7, 9-dioxaspiro[4.5]dec-1-enes 6 and 7. It might be due to the neighboring 2-aryl group and 3-methoxycarbonyl group has little steric hindance, both cis/trans-isomers 6 and 7 were formed in the sequential reaction process. At present the exact reason for the reaction proceeded via path a or path b are not clear, which would depend on the slightly different nucleophilic reactivity of arylidene Meldrum acids to that of arylidene N, N'-dimethylbarbituric acids.
|
Download:
|
| Scheme 1. Proposed reaction mechanism for three-component reaction. | |
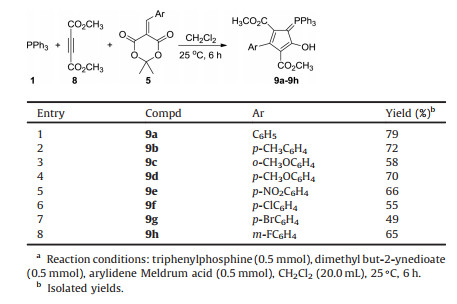
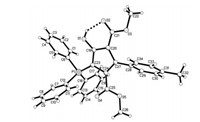
For developing the molecular diversity of this three-component reaction, another common electron-deficient alkyne, dimethyl but-2-ynedioate 8, was also employed in the reaction. The threecomponent reaction of triphenylphosphine, dimethyl but-2- ynedioate and arylidene Meldrum acids was also carried out in methylene dichloride at room temperature. Steading of formation of the expected spiro compound, the polysubstituted 5-(triphenyl-λ5-phosphanylidene)cyclopentadienes 9a-9h was obtained in satisfactory yields (Table 3). The structures of the compounds 9a-9h were confirmed by determination of the three single crystals of 9b (Fig. 4), 9d and 9e (Figs. S6 and S7 in Supporting information). Crystallographic data 9b (CCDC No. 1907406), 9d (CCDC No. 1907407), and 9e (CCDC No. 1907408) have been deposited at the Cambridge Crystallographic Data base Centre. The reaction did not result in the formation of the expected spiro compounds, while the ring of Meldrum acid is opened in the reaction and a polysubstituted cyclopentadiene was constructed. From the single crystal structures, it can be seen that the ring of cyclopentadiene was in one plane and an H-bond exists between the hydroxyl group and the carbonyl group. It has been reported that triphenylphosphine promoted reaction of dimethyl but-2-ynedioate with arylidene N, N'-dimethylbarbituric acid afforded 2H-pyrano[2, 3-d]pyrimidine derivatives and the highly substituted dimethyl 2-[(1, 3-dimethyl-2, 4, 6-trioxotetrahydropyrimidin-5(2H)-ylidene)arylmethyl]-2- butenedioate under neutral conditions [68]. These results again showed that the nucleophilic phosphine-catalyzed reaction has very interesting molecular diversities.
|
|
Table 3 Synthesis of (triphenyl-λ5-phosphanylidene)cyclopentadienes 9a-9h.a |
|
Download:
|
| Fig. 4. Single crystal structure of the compound 9b. | |
For explaining the formation of the polysubstituted 5-(triphenyl-λ5-phosphanylidene)cyclopentadienes 9a-9h, a modified reaction mechanism was proposed in Scheme 2. At first, triphenylphosphine added to dimethyl but-2-ynedioate gave the active intermediate G, which in turn added to the benzylidene Meldrum acid to give the intermediate H. Then, the intramolecular substitution of carbanion to methoxide ion produced the cyclic intermediate I. The nucleophilic addition of the methoxide ion to the carbonyl group caused the ring-opening of Meldrum acid to form the intermediate J with elimination of acetone. Finally, the polysubstituted cyclopentadiene 9 was formed by decarboxylation process. The formation of the unstable spiro compound I with a triphenylphosphine cation might be the reason for the different results of dimethyl acetylenedicarboxylate to that of dimethyl hex- 2-en-4-ynedioate in the three-component reaction.

|
Download:
|
| Scheme 2. Proposed formation reaction for the compound 9a-9h. | |
In summary, we have investigated the three-component reactions of triphenylphosphine, electron-deficient alkynes and active 1, 1-diactivated alkenes such as arylidene Meldrum acids and N, N'-dimethylbarbituric acids. This reaction provided efficient synthetic protocols for the polysubstituted spiro compounds and cyclopentadiene derivatives. The most feature of this reaction is that the structurally similar arylidene Meldrum acids and N, N'- dimethylbarbituric acids displayed different regioselectivity and diastereoselectivity in the three-component reactions. It is interesting to find that triphenylphosphane not only acted as nucleophilic catalyst, but also as 5-(triphenyl-λ5-phosphanylidene)ylidene scaffold retaining in the final product. The reaction has the advantages of using readily available reagents, mild reaction conditions, satisfactory yields, and molecular diversity with high regioselectivity and diastereoselectivity. The potential applications of this multicomponent reaction in synthetic and medicinal chemistry might be quite significant.
Declaration of competing interestWe declared there is no conflict of interest in this paper.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.10.042.
| [1] |
C. Zhang, X. Lu, J. Org. Chem. 60 (1995) 2906-2908. DOI:10.1021/jo00114a048 |
| [2] |
X. Lu, C. Zhang, Z. Xu, Acc. Chem. Res. 34 (2001) 535-544. DOI:10.1021/ar000253x |
| [3] |
J.L. Methot, W.R. Roush, Adv. Synth. Catal. 346 (2004) 1035-1050. DOI:10.1002/adsc.200404087 |
| [4] |
Y. Wei, M. Shi, Acc. Chem. Res. 43 (2010) 1005-1018. DOI:10.1021/ar900271g |
| [5] |
H.C. Guo, Y.C. Fan, Z.H. Sun, Chem. Rev. 118 (2018) 10049-10293. DOI:10.1021/acs.chemrev.8b00081 |
| [6] |
L.W. Ye, J. Zhou, Y. Tang, Chem. Soc. Rev. 37 (2008) 1140-1152. DOI:10.1039/b717758e |
| [7] |
B.J. Cowen, S.J. Miller, Chem. Soc. Rev. 38 (2009) 3102-3116. DOI:10.1039/b816700c |
| [8] |
R. Zhou, R.F. Liu, R.F. Li, Z.J. He, Chin. J. Org. Chem. 34 (2014) 2385-2405. DOI:10.6023/cjoc201406049 |
| [9] |
P. Xie, Y. Huang, Org. Biomol. Chem. 13 (2015) 8578-8595. DOI:10.1039/C5OB00865D |
| [10] |
R. Zhou, Z. He, Eur. J. Org. Chem. (2016) 1937-1954. |
| [11] |
Y. Wei, M. Shi, Org. Chem. Front. 4 (2017) 1876-1890. DOI:10.1039/C7QO00285H |
| [12] |
Y.N. Gao, M. Shi, Chin. Chem. Lett. 28 (2017) 493-502. DOI:10.1016/j.cclet.2016.12.001 |
| [13] |
P. Karanam, G.M. Reddy, S.R. Koppolu, W. Lin, Tetrahedron Lett. 59 (2018) 59-76. DOI:10.1016/j.tetlet.2017.11.051 |
| [14] |
J.E. Wilson, G.C. Fu, Angew. Chem. Int. Ed. 45 (2006) 1426-1429. DOI:10.1002/anie.200503312 |
| [15] |
D.J. Wallace, R.L. Sidda, R.A. Reamer, J. Org. Chem. 72 (2007) 1051-1054. DOI:10.1021/jo062170l |
| [16] |
Y.Q. Fang, E.N. Jacobsen, J. Am. Chem. Soc. 130 (2008) 5660-5661. DOI:10.1021/ja801344w |
| [17] |
H. Xiao, Z. Chai, C.W. Zheng, et al., Angew. Chem. Int. Ed. 49 (2010) 4467-4470. DOI:10.1002/anie.201000446 |
| [18] |
A. Voituriez, N. Pinto, M. Neel, et al., Chem. -Eur. J. 16 (2010) 12541-12544. DOI:10.1002/chem.201001791 |
| [19] |
L.W. Ye, X.L. Sun, Q.G. Wang, et al., Angew. Chem. Int. Ed. 46 (2007) 5951-5954. DOI:10.1002/anie.200701460 |
| [20] |
S. Zheng, X. Lu, Org. Lett. 11 (2009) 3978-3981. DOI:10.1021/ol901618h |
| [21] |
R. Baharfar, M.J. Taghizadeh, M. Ahmadian, et al., Chin. Chem. Lett. 21 (2010) 175-178. DOI:10.1016/j.cclet.2010.02.012 |
| [22] |
M.H. Mosslemin, N. Shams, H. Esteghamat, et al., Chin. Chem. Lett. 24 (2013) 1095-1098. DOI:10.1016/j.cclet.2013.06.031 |
| [23] |
Y.Q. Jiang, Y.L. Shi, M. Shi, J. Am. Chem. Soc. 130 (2008) 7202-7203. DOI:10.1021/ja802422d |
| [24] |
S. Xu, L. Zhou, S. Zeng, et al., Org. Lett. 11 (2009) 3498-3501. DOI:10.1021/ol901334c |
| [25] |
Z. Chen, J. Zhang, Chem. -Asian J. 5 (2010) 1542-1545. DOI:10.1002/asia.201000193 |
| [26] |
P. Xie, Y. Huang, R. Chen, Org. Lett. 12 (2010) 3768-3771. DOI:10.1021/ol101611v |
| [27] |
S. Xu, L. Zhou, R. Ma, H. Song, Z. He, Org. Lett. 12 (2010) 544-547. DOI:10.1021/ol902747c |
| [28] |
S. Xu, W. Zou, G. Wu, H. Song, Z. He, Org. Lett. 12 (2010) 3556-3559. DOI:10.1021/ol101429z |
| [29] |
F. Zhong, X. Han, Y. Wang, Y. Lu, Angew. Chem. Int. Ed. 50 (2011) 7837-7841. DOI:10.1002/anie.201102094 |
| [30] |
X.C. Zhang, S.H. Cao, M. Shi, Org. Lett. 13 (2011) 1142-1145. DOI:10.1021/ol1031798 |
| [31] |
H. Deng, Y. Wei, M. Shi, Org. Lett. 13 (2011) 3348-3351. DOI:10.1021/ol201094f |
| [32] |
X. Zhang, S. Cao, Y. Wei, M. Shi, Chem. Commun. 47 (2011) 1548-1550. DOI:10.1039/C0CC04289G |
| [33] |
R. Zhou, C.J. Yang, Y.Y. Liu, et al., J. Org. Chem. 79 (2013) 10709-10715. |
| [34] |
Z.F. Qin, W. Liu, D.Y. Wang, et al., J. Org. Chem. 81 (2016) 4690-4700. DOI:10.1021/acs.joc.6b00596 |
| [35] |
C.J. Yang, X.Y. Chen, T. Tang, et al., J. Org. Lett. 18 (2016) 1486-1489. DOI:10.1021/acs.orglett.6b00456 |
| [36] |
C.Z. Zhu, Y.L. Sun, Y. Wei, M. Shi, Adv. Synth. Catal. 359 (2017) 1263-1270. DOI:10.1002/adsc.201700031 |
| [37] |
D.B. Ramachary, T.P. Reddy, A. Suresh Kumar, Org. Biomol. Chem. 15 (2017) 9785-9789. DOI:10.1039/C7OB02424J |
| [38] |
J. Zhang, H.H. Wu, J. Zhang, Org. Lett 19 (2017) 6080-6083. DOI:10.1021/acs.orglett.7b02895 |
| [39] |
C. Wang, Z. Gao, L. Zhou, et al., Chem. Commun 54 (2018) 279-282. DOI:10.1039/C7CC08380G |
| [40] |
G. Zhan, M.L. Shi, Q. He, et al., Angew. Chem. Int. Ed. 55 (2016) 2147-2151. DOI:10.1002/anie.201510825 |
| [41] |
L. Zhou, C. Yuan, C. Zhang, et al., Adv. Synth. Catal. 359 (2017) 2316-2321. DOI:10.1002/adsc.201601434 |
| [42] |
L. Li, L. Ye, S.F. Ni, et al., Org. Chem. Front. 4 (2017) 2139-2146. DOI:10.1039/C7QO00500H |
| [43] |
X.Y. Zhang, S.X. Kang, Y.M. Gao, et al., Chin. Chem. Lett. 21 (2010) 312-316. DOI:10.1016/j.cclet.2009.11.040 |
| [44] |
R. Ghorbani-Vaghei, L. Shiri, A. Ghorbani-Choghamarani, Chin. Chem. Lett. 24 (2013) 1123-1126. DOI:10.1016/j.cclet.2013.07.020 |
| [45] |
L.Y. Cui, S.H. Guo, B. Li, X.Y. Zhang, et al., Chin. Chem. Lett. 25 (2014) 55-57. DOI:10.1016/j.cclet.2013.10.008 |
| [46] |
H.Y. Duan, J. Ma, Z.Z. Yuan, et al., Chin. Chem. Lett. 26 (2015) 646-648. DOI:10.1016/j.cclet.2015.03.019 |
| [47] |
K.K. Wang, W. Du, J. Zhu, et al., Chin. Chem. Lett. 28 (2017) 512-516. DOI:10.1016/j.cclet.2016.11.003 |
| [48] |
S. Li, Y. Liu, B. Huang, et al., ACS Catal. 7 (2017) 2805-2809. DOI:10.1021/acscatal.7b00030 |
| [49] |
P.V. Ramachandran, M.T. Rudd, M.V.R. Reddy, Tetrahedron Lett. 46 (2005) 2547-2549. DOI:10.1016/j.tetlet.2005.02.098 |
| [50] |
L.H. Zhou, X.Q. Yu, L. Pu, Tetrahedron Lett. 51 (2010) 425-427. DOI:10.1016/j.tetlet.2009.11.048 |
| [51] |
S.F. Duan, D.K. Sinha-Mahapatra, J.W. Herndon, Org. Lett. 10 (2008) 1541-1544. DOI:10.1021/ol800242n |
| [52] |
F. Puenner, G. Hilt, Chem. Commun. 48 (2012) 3617-3619. DOI:10.1039/c2cc30777d |
| [53] |
S. Raju, P. Annamalai, F.W. Chan, et al., Synthesis 49 (2017) 5007-5016. DOI:10.1055/s-0036-1588501 |
| [54] |
S.F. Jin, Y.J. Niu, C.J. Liu, et al., J. Org. Chem. 82 (2017) 9066-9074. DOI:10.1021/acs.joc.7b01561 |
| [55] |
S. Sakthivel, A. Sharma, R. Balamurugan, Eur. J. Org. Chem. (2017) 3941-3946. |
| [56] |
B. Dhakal, L.S.R. Gamage, Y.S. Zhang, J.W. Herndon, Tetrahedron Lett. 58 (2017) 1403-1407. DOI:10.1016/j.tetlet.2017.02.070 |
| [57] |
J.H. Choi, C.M. Park, Adv. Synth. Catal. 360 (2018) 3553-3562. DOI:10.1002/adsc.201800734 |
| [58] |
J.C. Deng, S.C. Chuang, Org. Lett. 13 (2011) 2248-2251. DOI:10.1021/ol200527t |
| [59] |
P.Y. Tseng, S.C. Chuang, Adv. Synth. Catal. 355 (2013) 2165-2171. DOI:10.1002/adsc.201300255 |
| [60] |
J.C. Deng, W.Y. Chen, C.Y. Zhu, et al., Adv. Synth. Catal. 357 (2015) 1453-1462. DOI:10.1002/adsc.201401134 |
| [61] |
A.J. Wu, P.Y. Tseng, W.H. Hsu, et al., Org. Lett. 18 (2016) 224-227. DOI:10.1021/acs.orglett.5b03293 |
| [62] |
Y. Han, Y.J. Sheng, C.G. Yan, Org. Lett. 16 (2014) 2654-2657. DOI:10.1021/ol5008394 |
| [63] |
H. Gong, J. Sun, C.G. Yan, Synthesis 46 (2014) 2327-2332. DOI:10.1055/s-0033-1339120 |
| [64] |
Y. Han, W.J. Qi, Y.J. Shen, et al., Tetrahedron Lett. 56 (2015) 5196-5198. DOI:10.1016/j.tetlet.2015.07.071 |
| [65] |
W.J. Qi, Y. Han, C.G. Yan, Tetrahedron 72 (2016) 5057-5063. DOI:10.1016/j.tet.2016.06.020 |
| [66] |
C.Z. Liu, Y. Han, W.J. Qi, et al., Heterocyclic Commun. 22 (2016) 301-306. DOI:10.1093/jac/dks075 |
| [67] |
W.J. Qi, Y. Han, C.Z. Liu, et al., Chin. Chem. Lett. 28 (2017) 442-445. DOI:10.1016/j.cclet.2016.09.014 |
| [68] |
M. Bayat, Y. Bayat, S.S. Asayesh, Monatsh. Chem. 143 (2012) 479-483. DOI:10.1007/s00706-011-0602-7 |
 2020, Vol. 31
2020, Vol. 31