b State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing 100191, China;
c University of Chinese Academy of Sciences, Beijing 100049, China;
d School of Pharmaceutical Science, Shanxi Medical University, Taiyuan 030001, China
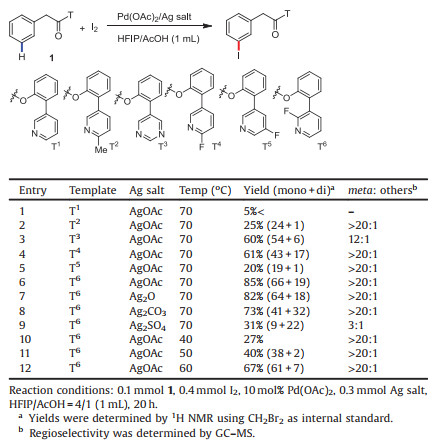
Aryl iodides are widely utilized as important starting materials in organic chemistry and pharmaceutical drug syntheses [1]. Many synthetic methods have been developed for the construction of aryl iodide, and metal-catalyzed C—H iodination of arene is the most straightforward way [2]. Assisted by a directing group, Pd [3], Rh [4], Ru [5], Cu [6], Ni [7], and Co [8] have been adopted in the ortho-C—H iodination of arenes. Athough Pd-catalyzed meta-C—H chlorination using norbornene as the mediator and Ru-catalyzed meta-C—H bromination via electronic effects have been reported [9, 10], meta-C—H iodination has been less explored. The rational design of a directing group with proper distance and geometry plays an important role to recognize the remote C—H bonds [11, 12]. Recently, Yu elegantly designed pyridine-containing templates and realized the palladium-catalyzed meta-C—H iodination of benzyl and phenyl ethyl alcohols by using 1, 3- diiodo-5, 5-dimethylhydantoin (DIH) as the iodinating source [13] (Scheme 1a). Due to the high importance of the phenylacetic acid in organic synthesis and drug molecules, Yu subsequently applied this strategy to phenylacetic acid with the template anchoring into the substrates through an amide linkage [14] (Scheme 1b).
|
Download:
|
| Scheme 1. Directed C—H iodinaion. | |
Using the inexpensive and milder molecular I2 as iodinating reagent has become an attractable alternative in metal-catalyzed C—H iodination. In 2013, Yu reported the Pd-catalyzed ortho-C—H iodination of benzamides and phenylacetic amides using I2 as the sole oxidant [3a]. Subsequently, Chatani and Koley realized the Nicatalyzed ortho-C—H iodination of benzamides with molecular I2 [7]. Very recently, Chatani reported Co-catalyzed chelationassisted ortho-C—H iodination of benzamides with molecular I2 as an iodinating reagent and Ag2CO3 as the oxidant [8]. Prompted by these reports and our recent achievement in meta-C—H deuteration of arenes [15], we questioned whether we could realize the meta-C—H iodination by using molecular I2 as the iodinating source. Herein, we reported the template-directed meta-C—H iodination of phenylacetic acid, benzylphosphonate, and benzylsulfonate scaffolds by using molecular I2 (Scheme 1c). Considering that the directing group should be easily installed and readily removed for bioactive molecules, we anchored the template into the substrates through a practical ester linkage.
We began our investigation by testing the meta-C—H iodination of phenylacetic acid containing a variety of 3-subsituted pyridine based templates (Table 1). When simple pyridyl moiety in substrate 1 is subjected to meta-C—Hiodination, only < 5%yields of iodination product could be formed (entry 1). It was possible that the coordination of pyridine with Pd(Ⅱ) was too strong to form reactive complex. Introduce a methyl group at 6 position could improve the yields slightly to 25% (entry 2). Pyrimidine-based template has been recently explored by Maiti in Pd-catalyzed meta-C–H functionalizations [12]. When we change the heterocycle from pyridine to pyrimidine, the yields improve to 60% with 54% mono and 6% di (entry 3). Tuning the coordination ability by incorporation an electron-withdrawing fluoro group at the C-2 and C-6 position of the pyridine ring lead to improved yields, and 2-fluoro-3-pyridyl template T6 showed the highest yield and meta-selectivity (entries 4–6). Among other silver salts, Ag2O showed comparable results to AgOAc with 82% yields (entries 7–9). Lowering the reaction temperature lead to the decreased yield of iodinated product (entries 10–12).
|
|
Table 1 Optimization of the reaction conditions for meta-C—H Iodination. |
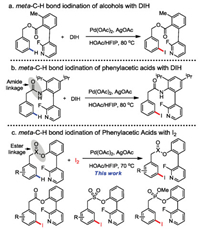
With the optimized reaction conditions in hand, we turned our attention to investigate the phenylacetic acid scope (Scheme 2). meta-C—H iodination of phenylacetic acid provided the desired products 2a in 81% isolated yields (62% mono and 19% di) with high regioselectivity. Phenylacetic acid containing electron-donating methyl, methoxyl group at the 2-, 3- or 4-position position could be iodinated to give the corresponding products in moderate to good yields with excellect meta ratio (2b, 2c, 2f, 2j). It is worth noting that substrates containing electron-withdrawing fluoro, chloro, iodo and even trifluoromethyl groups could react with I2, giving the corresponding products with good to moderate yields (2d, 2e, 2g, 2i, 2k, 2l). The halide groups in products are useful handle for further structural elaborations. For 2, 3- or 2, 4- disubstituted phenylacetic acids, C—H iodination occurred mainly at the less hindered positions with moderate to good yields (2m-2o). Furthermore, α-alkyl, cycloalkyl, heteroatom substituted phenylacetic acids were also compatible in this reaction (2p-2s, 2w, 2x). The versatility of this protocol was further validated by meta-C—H iodination of cyclic substrates (2u, 2v). Ibuprofen derivative can also be meta-iodinated to give the corresponding mono iodinated product in 60% yield with meta/others > 20/1 (2t). The regioselectivity of product 2t was determined by NOE analysis.
|
Download:
|
| Scheme 2. Scope of phenylacetic acids for meta-C—H iodination. Reaction conditions: 1 (0.1 mmol), 0.4 mmol I2, 10 mol% Pd(OAc)2, 0.3 mmol AgOAc, HFIP/ AcOH = 4/1 (1 mL), 70 ℃, 20 h. Mono and di are showed in parenthesis. Regioselectivity was determined GC–MS. a 0.3 mmol I2. b 80 ℃. c 0.2 mmol I2. d 20 mol% N-Ac-Gly-OH was added as ligands. e 100 ℃. f Regioselectivity was determined LC–MS. | |
Benzylphosphonate and benzylsulfonate substrates are common structural motifs and useful synthons in organic chemistry [16]. To demonstrate the broad utility of this methodology, we subjected the substrates 1y and 1z to the meta-C—H iodination conditions. The corresponding product 2y and 2z could be obtained in 56% and 58% yields respectively (Scheme 3).

|
Download:
|
| Scheme 3. meta-C—H iodination of benzylphosphonate and benzylsulfonate. | |
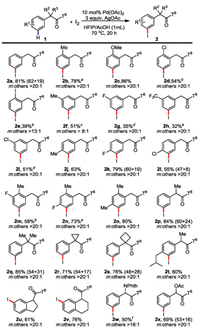
We also applied this protocol for gram-scale reaction using ibuprofen derivative 1t under the standard condition, giving the desired product 2t in 73% isolated yields (Scheme 4).
|
Download:
|
| Scheme 4. Gram-scale reaction. | |
meta-C—H iodinated products are versatile step stone for further functionalization at meta positions of arenes. For example, iodinated ibuprofen derivative 2t could be converted to a diverse range of meta-substituted ibuprofen ester by alcoholysis and subsequently Pd-catalyzed cross-coupling reactions, illustrating the broad utility of this methodology (Scheme 5).
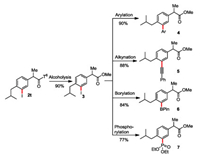
|
Download:
|
| Scheme 5. Transformation of iodinated ibuprofen derivative 2t. | |
Finally, the template could be easily removed by hydrolysis of 2c at room temperature by using LiOH in THF/H2O, giving the meta-iodinated phenylacetic acids 8 in good yields (Scheme 6).

|
Download:
|
| Scheme 6. Removal of the directing template. | |
In summary, we developed Pd-catalyzed highly meta-selective C—H iodination of phenylacetic acid, benzylphosphonate, and benzylsulfonate scaffolds using a pyridine-type template. The directing template was anchored to the substrates via the practical ester linkage. A variety of phenylacetic acids containing electronwithdrawing and electron-donating substituents are compatible with these reactions. Further transformations of ibuprofen iodide intermediates by Pd-catalyzed C—C and C–heteroatom bond formation demonstrated the importance of this methodology in organic synthesis and drug discovery.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe gratefully acknowledge Shanghai Institute ofMateriaMedica, Chinese Academy of Sciences, National Natural Science Foundation of China (No. 21772211), Youth Innovation Promotion Association CAS (Nos. 2014229 and 2018293), Institutes for Drug Discovery and Development, Chinese Academy of Sciences (No. CASIMM0120163006), Science and Technology Commission of Shanghai Municipality (No. 17JC1405000), Program of Shanghai Academic Research Leader (No. 19XD1424600), National Science & Technology Major Project "Key New Drug Creation and Manufacturing Program", China (No. 2018ZX09711002-006), and the State Key Laboratory of Natural and Biomimetic Drugs for financial support.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.057.
| [1] |
(a) P.Ruiz-Castillo, S.L.Buchwald, Chem.Rev.116 (2016)12564-12649; (b) F.S.Han, Chem.Soc.Rev.42 (2013)5270-5298; (c) G.Evano, N.Blanchard, M.Toumi, Chem.Rev.108 (2008)3054-3131; (d) D.Ma, Q.Cai, Acc.Chem.Res.41 (2008)1450-1460. |
| [2] |
D.A. Petrone, J. Ye, M. Lautens, Chem.Rev. 116 (2016) 8003-8104. DOI:10.1021/acs.chemrev.6b00089 |
| [3] |
(a) X.C.Wang, Y.Hu, S.Bonacorsi, et al., J.Am.Chem.Soc.135 (2013)10326-10329; (b) L.Chu, X.C.Wang, C.E.Moore, A.L.Rheingold, J.Q.Yu, J.Am.Chem.Soc.135 (2013)16344-16347; (c) L.Chu, K.J.Xiao, J.Q.Yu, Science 346 (2014)451-455; (d) J.J.Li, T.S.Mei, J.Q.Yu, Angew.Chem.Int.Ed.47 (2008)6452-6455; (e) R.Giri, X.Chen, J.Q.Yu, Angew.Chem.Int.Ed.44 (2005)2112-2125; (f) D.Kalyani, A.R.Dick, W.Q.Anani, M.S.Sanford, Org.Lett.8 (2006)2523-2526. |
| [4] |
(a) N.Schröder, F.Lied, F.Glorius, J.Am.Chem.Soc.137 (2015)1448-1451; (b) H.Hwang, J.Kim, J.Jeong, S.Chang, J.Am.Chem.Soc.136 (2014)10770-10776; (c) N.Schröder, J.Wencel-Delord, F.Glorius, J.Am.Chem.Soc.134 (2012)8298-8301. |
| [5] |
L. Wang, L. Ackermann, Chem.Commun. 50 (2014) 1083-1085. DOI:10.1039/C3CC47852A |
| [6] |
(a) B.Yao, Z.L.Wang, H.Zhang, et al., J.Org.Chem.77 (2012)3336-3340; (b) B.Li, B.Liu, B.F.Shi, Chem.Commun.51 (2015)5093-5096; (c) X.Chen, X.S.Hao, C.E.Goodhue, J.Q.Yu, J.Am.Chem.Soc.128 (2006)6790-6791. |
| [7] |
(a) Y.Aihara, N.Chatani, ACS Catal.6 (2016)4323-4329; (b) B.Khan, R.Kant, D.Koley, Adv.Synth.Catal.358 (2016)2352-2358. |
| [8] |
(a) Y.Kommagalla, K.Yamazaki, T.Yamaguchi, N.Chatani, Chem.Commun.54 (2018)1359-1362; (b) Y.Kommagalla, N.Chatani, Org.Lett.21 (2019)5971-5976. |
| [9] |
H. Shi, P. Wang, S. Suzuki, M.E. Farmer, J.Q. Yu, J. Am. Chem. Soc. 138 (2016) 14876-14879. DOI:10.1021/jacs.6b11055 |
| [10] |
(a) C.J.Teskey, A.Y.W.Lui, M.F.Greaney, Angew.Chem.Int.Ed.54 (2015)11677-11680; (b) Q.Yu, L.Hu, Y.Wang, S.Zheng, J.Huang, Angew.Chem.Int.Ed.54 (2015)15284-15288; (c) S.Warratz, D.J.Burns, C.Zhu, etal., Angew.Chem.Int.Ed.56 (2017)1557-1560. |
| [11] |
(a) Y.F.Yang, X.Hong, J.Q.Yu, K.N.Houk, Acc.Chem.Res.50 (2017)2853-2860; (b) A.Dey, S.K.Sinha, T.K.Achar, D.Maiti, Angew.Chem.Int.Ed.58 (2019)10820-10843; (c) A.Dey, S.Agasti, D.Maiti, Org.Biomol.Chem.14(2016)5440-5453; (d) Y.Gao, G.Li, Aldrichimica Acta 50 (2017)61-73. |
| [12] |
(a) D.Leow, G.Li, T.S.Mei, J.Q.Yu, Nature 486 (2012)518-522; (b) R.Jayarajan, J.Das, S.Bag, R.Chowdhury, D.Maiti, Angew.Chem.Int.Ed.57 (2018)7659-7663; (c) L.Fang, T.G.Saint-Denis, K.N.Houk, J.Q.Yu, J.Am.Chem.Soc.139 (2017)10702-10714; (d) S.Bag, R.Jayarajan, U.Dutta, et al., Angew.Chem.Int.Ed.56 (2017)12538-12542; (e) S.Li, L.Cai, H.Ji, L.Yang, G.Li, Nat.Commun.7 (2016)10443; (f) A.Modak, T.Patra, R.Chowdhury, S.Raul, D.Maiti, Organometallics 36 (2017)2418-2423; (g) S.Bag, T.Patra, A.Modak, et al., J.Am.Chem.Soc.137 (2015)11888-11891; (h) M.Bera, A.Maji, S.K.Sahoo, D.Maiti, Angew.Chem.Int.Ed.54 (2015)8515-8519; (i) R.Tang, G.Li, J.Q.Yu, Nature 507(2014)215-220; (j) S.Lee, H.Lee, K.L.Tan, J.Am.Chem.Soc.135 (2013)18778-18781; (k) L.Zhang, C.Zhao, Y.Liu, et al., Angew.Chem.Int.Ed.56 (2017)12245-11249; (l) J.Xu, J.Chen, F.Gao, et al., J.Am.Chem.Soc.141 (2019)1903-1907; (m) H.J.Xu, Y.Lu, M.E.Farmer, et al., J.Am.Chem.Soc.139 (2017)2200-2203; (n) H.J.Xu, Y.S.Kang, H.Shi, et al., J.Am.Chem.Soc.141 (2019)76-79; (o) R.J.Phipps, M.J.Gaunt, Science 329 (2009)1593-1597; (p) Y.Kuninobu, H.Ida, M.Nishi, M.Kanai, Nat.Chem.7 (2015)712-717; (q) H.L.Li, Y.Kuninobu, M.Kanai, Angew.Chem.Int.Ed.56 (2017)1495-1499; (r) R.Bisht, B.Chattopadhyay, J.Am.Chem.Soc.138 (2016)84-87; (s) H.J.Davis, M.T.Mihai, R.J.Phipps, J.Am.Chem.Soc.138 (2016)12759-12762; (t) H.J.Davis, G.R.Genov, R.J.Phipps, Angew.Chem.Int.Ed.56 (2017)13351-13355; (u) M.E.Hoque, R.Bisht, C.Haldar, B.Chattopadhyay, J.Am.Chem.Soc.139 (2017)7745-7748; (v) M.Li, M.Shang, H.Xu, et al., Org.Lett.21 (2019)540-544. |
| [13] |
L. Chu, M. Shang, K. Tanaka, et al., ACS Cent.Sci. 1 (2015) 394-399. DOI:10.1021/acscentsci.5b00312 |
| [14] |
Z. Jin, L. Chu, Y.Q. Chen, J.Q. Yu, Org.Lett. 20 (2018) 425-428. |
| [15] |
H. Xu, M. Liu, L.J. Li, et al., Org.Lett. 21 (2019) 4887-4891. DOI:10.1021/acs.orglett.9b01784 |
| [16] |
(a) M.Bera, S.K.Sahoo, D.Maiti, ACS Catal.6 (2016)3575-3579; (b) X.Meng, S.Kim, Org.Lett.15 (2013)1910-1913; (c) W.J.Stec, Acc.Chem.Res.16 (1983)411-417; (d) J.Boutagy, R.Thomas, Chem.Rev.74 (1974)87-99; (e) W.S.Wadsworth, W.D.Emmons, J.Am.Chem.Soc.83 (1961)1733-1738; (f) G.Cheng, P.Wang, J.Q.Yu, Angew.Chem.Int.Ed.56 (2017)8183-8186. |
 2020, Vol. 31
2020, Vol. 31