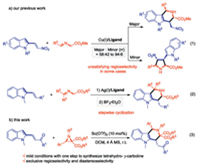
Tetrahydro-γ-carboline derivatives featuring various biological properties have received an intense focus among medical chemists [1]. Efficient synthetic routes to this scaffold are still intensively pursued. Intramolecular cyclizations of functionalized indole derivatives for the synthesis of tetrahydro-γ-carbolines have received great attentions, including iso-Pictet-Spengler reaction [2] and Pd-catalyzed intramolecular alkylation [3]. In contrast, direct access to highly-substituted tetrahydro-γ-carbolines via the intermolecular cycloadditions of indolyl synthons still remained in highly desirable [4, 5]. Considering the unique advantage of [3+3] cycloadditions in the rapid construction of diverse six-member ring structures [6-11], we recently reported copper-catalyzed asymmetric [3+3] cycloaddition of 2-indolylnitroethylenes with azomethine ylides [12], generated from aldimino esters, to construct highly substituted tetrahydro-γ-carbolines in moderate to high yields and an excellent level of stereoselectivity (Scheme 1, Eq. (1)). However, the transformation is companied with an inevitable competition between [3+2] cycloaddition and [3+3] cycloaddition, which displays an unsatisfying regioselectivity in some cases. Subsequently, the ketones derived azomethine ylides were employed in the reaction to prevent the competitive [3+2] cycloaddition and delivered the tetrahydro-γ-carbolines in excellent regioselectivities, however, a stronger Lewis acid BF3Et2O was essential for the cyclization (Scheme 1, Eq. (2)) [13, 14].
|
Download:
|
| Scheme 1. [3+3] cycloadditions to synthesize tetrahydro-γ-carbolines. | |
Donor-acceptor (D-A) aziridines, as potent precursors of azomethine ylides, prefer the C—C bond cleavage in the presence of Lewis acids, which can further undergo cycloadditions with various dipolarphiles [15-30]. Compared to the well-developed [3+2] cycloadditions with two-atom dipolarphiles, [3+3] cycloadditions with three-atom moieties were still merely reported by Banerjee [31] and Kim [32, 33]. In the context, we envisage selecting the 2, 2'-diester aziridines as the precursors of azomethine ylides, which are attacked by the nucleophilic C3 position of the indole ring followed by an intermolecular Michael addition, to construct the biologically important tetrahydro-γ-carboline skeletons in one-step process (Scheme 1, Eq. (3)).
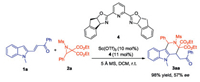
We initiated the present investigation of β-(indol-2-yl)-α, β-unsaturated ketone 1a (1.0 equiv.) with 2, 2'-diester aziridine 2a (1.5 equiv.) in the presence of a catalytic amount of Sc(OTf)3 (10 mol%) as the Lewis acid catalyst in DCM at room temperature. Since 2a was the moisture sensitive compound, activated 4 Å molecular sieves (MS) were added. To our delight, the target product tetrahydro-γ-carboline 3aa was generated in 96% yield with a single diastereoisomer (Table 1, entry 1). Subsequently, various Lewis acids were examined to optimize the reaction conditions (Table 1, entries 1–6), and Sc(OTf)3 was selected as the optimal Lewis acid. Then, the effect of MS was evaluated (Table 1, entries 7 and 8). The absence of MS caused a diminished yield (71% yield), while switching to 5 Å MS led to the reaction time extending from 0.5 h to 2 h. Thus, 4 Å MS was chosen as an optimal additive. Examination of solvent effect indicated that the reaction proceeds better in a chlorinated solvent considering yield and reaction time (Table 1, entries 9–11), with DCM as the best choice. Finally, by reducing the amount of the catalyst, the product was obtained with a decreased rate and a lowered yield (Table 1, entries 12 and 13). Therefore, we chose 10 mol% Sc(OTf)3/4 Å MS/DCM as the optimal reaction conditions.
|
|
Table 1 Optimization of reaction conditions.a |
With optimized reaction conditions in hand, we embarked on the investigation of several β-(indol-2-yl)-α, β-unsaturated ketones with a variety of aryl and alkyl substituents. As summarized in Table 2, various tetrahydro-γ-carbolines were obtained in good to excellent yields. Introducing groups onto the para-situation of the phenyl ring, reactions gave the desired products in a yield of 61%– 84% (3ba-3fa). Halogens such as Cl and Br, electron withdrawing groups such as CN, and electron donating groups such as CH3 and CH3O are tolerated in this transformation. The ortho-Bromo or meta-Bromo substituent does not affect the reaction result (3ga, 3ha). Substrates with 2-thienyl and 2-naphthyl are also suitable for this reaction (3ia, 3ja). When the phenyl ring on the indole derivatives was changed to n-butyl, the product 3ka was obtained smoothly in a good yield.
|
|
Table 2 Optimization of substrate scope of β-(indol-2-yl)-α, β-unsaturated ketones.a |
Encouraged by these results, we further probed the reaction scope of the 2, 2'-diester aziridines with different substituents. As illustrated in Table 3, aziridines were broadly tolerated with reasonable scope, irrespective of the electronic nature of the substituents on the phenyl ring (3ab-3ai). The structure and the relative stereochemistry of 3ag were determined unambiguously by single crystal X-ray crystallographic analysis where phenyl and benzoylmethylene groups were found in the cis configuration (Supporting information for details). Crystallographic data of 3ag (CCDC 1943454) can be obtained free of charge from the Cambridge Crystallographic Data Centre. N-Methylsulfonyl, 2, 2- dimethyl ester and 2, 2-diisopropyl ester substituted aziridines are also amenable to this transformation, providing corresponding products in good to excellent yields (3aj-3al).
|
|
Table 3 Substrate scope of 2, 2'-diester aziridines.a |
In the view of X-ray crystal results and literature reports [34], we outlined the plausible mechanism for this [3+3] cycloaddition in Scheme 2. To take the reaction between β-(indol-2-yl)-α, β- unsaturated ketone 1a and 2, 2'-diester aziridine 2a as an example, 2a first coordinates to Sc(OTf)3 to form intermediate I, which results in C—C bond cleavage to form azomethine ylide II. Subsequently, a stepwise [3+3] cycloaddition occurs. The C3 position of indole nucleophile attacks the N-Ms iminium carbon, delivering intermediate III. By attacking the tethered activated olefin of its malonate anion moiety in Michael fashion, the following ring closing process gives rise to a Sc(OTf)3 coordinated polysubstituted tetrahydro-γ-carboline V. Compared with the Michael acceptor in pseudoequatorial position suffering a severe gauche interaction with two ester groups in conformer VI, Michael acceptor in pseudoaxial position only has an interaction with one ester moiety in conformer IV, which is the favored conformer, providing the tetrahydro-γ-carboline 3aa with the phenyl and benzoylmethylene groups in cis configuration. The final ligand exchange with 2a liberates the product 3aa and furnishes the catalytic cycle.

|
Download:
|
| Scheme 2. Mechanism of the reaction and origin of diastereoselectivity. | |
In principle, stereochemically enriched tetrahydro-γ-carboline derivatives of optical purity can be obtained by introducing the suitable chiral ligand onto the Lewis acid Sc(OTf)3. After screening of various chiral ligands (Supporting inforamtion for details), unfortunately, only moderate enantioselectivity (57% ee) was obtained by using the commercially available Pybox 4 as the ligand under reaction conditions depicted in Scheme 3. Although the enantioselectivity is not good enough as yet, it still manifests that an asymmetric reaction is feasible.
|
Download:
|
| Scheme 3. Catalytic enantioselective reaction to tetrahydro-γ-carbolines. | |
In conclusion, we have developed a mild and practical synthetic strategy to polysubstituted tetrahydro-γ-carbolines in one-step process. A wide range of tetrahydro-γ-carbolines were obtained in single diastereoisomers in good to excellent yields via Sc(OTf)3 catalyzed [3+3] cycloaddition of 2, 2'-diester aziridines with β- (indol-2-yl)-α, β-unsaturated ketones. In addition, the enantioselective version was also investigated, albeit with moderate ee value.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (No. 21772038) and the China Postdoctoral Science Foundation (No. 2018M632037).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2019.09.002.
| [1] |
J. Dai, W. Dan, Y. Zhang, J. Wang, Eur. J. Med. Chem. 157 (2018) 447-461. |
| [2] |
Y. Lee, R.S. Klausen, E.N. Jacobsen, Org. Lett. 13 (2011) 5564-4467. DOI:10.1021/ol202300t |
| [3] |
D. Sole, M.L. Bennasar, I. Jimenez, Org. Biomol. Chem. 9 (2011) 4535-4544. |
| [4] |
H.G. Cheng, C.B. Chen, F. Tan, et al., Eur. J. Org. Chem. 2010 (2010) 4976-4980. DOI:10.1002/ejoc.201000853 |
| [5] |
X.X. Sun, H.H. Zhang, G.H. Li, et al., Chem. -Eur. J. 22 (2016) 17526-17532. |
| [6] |
R.P. Hsung, A.V. Kurdyumov, N Sydorenko, Eur. J. Org. Chem. (2005) 23-44. DOI:10.1002/chin.200516251 |
| [7] |
X. Xu, M.P. Doyle, Acc. Chem. Res. 47 (2014) 1396-1405. DOI:10.1021/ar5000055 |
| [8] |
F. Shi, R.Y. Zhu, W. Dai, et al., Chem. -Eur. J. 20 (2014) 2597-2604. |
| [9] |
X.X. Sun, C. Li, Y.Y. He, et al., Adv. Synth. Catal. 359 (2017) 2660-2670. |
| [10] |
W. Dai, H. Lu, X. Li, et al., Chem. Eur. J. 20 (2014) 11382-11389. DOI:10.1002/chem.201402485 |
| [11] |
Z.Q. Zhu, L. Yu, M. Sun, et al., Adv. Synth. Catal. 36 (2018) 3109-3116. |
| [12] |
W.L. Yang, C.Y. Li, W.J. Qin, et al., ACS Catal. 6 (2016) 5685-5690. |
| [13] |
X. Zheng, W.L. Yang, Y.Z. Liu, et al., Adv. Synth. Catal. 360 (2018) 2843-2853. DOI:10.1002/adsc.201800553 |
| [14] |
Y.Z. Liu, S.J. Shang, J.Y. Zhu, et al., Adv. Synth. Catal. 360 (2018) 2191-2203. |
| [15] |
X. Wu, J. Zhang, Synthesis 44 (2012) 2147-2154. DOI:10.1055/s-0031-1290816 |
| [16] |
P.D. Pohlaus, R.K. Bowman, J.S. Johnson, J. Am. Chem. Soc. 126 (2004) 2294-2295. DOI:10.1021/ja0397963 |
| [17] |
L. Li, X. Wu, J. Zhang, Chem. Commun. 47 (2011) 5049-5051. DOI:10.1039/c1cc10926j |
| [18] |
X. Wu, L. Li, J. Zhang, Chem. Commun. 47 (2011) 7824-7826. DOI:10.1039/c1cc12189h |
| [19] |
L. Li, J. Zhang, Org. Lett. 13 (2011) 5940-5943. DOI:10.1021/ol202603e |
| [20] |
Z. Jiang, J. Wang, P. Lu, Y.G. Wang, Tetrahedron 67 (2011) 9609-9617. DOI:10.1016/j.tet.2011.09.085 |
| [21] |
R.A. Craig II, N.R. O'Connor, A.F. Goldberg, B.M. Stoltz, Eur. J. Chem. 20 (2014) 4806-4813. DOI:10.1002/chem.201303699 |
| [22] |
H. Liu, C. Zheng, S.L. You, Chin. J. Chem. 32 (2014) 709-714. DOI:10.1002/cjoc.201400178 |
| [23] |
T. Soeta, Y. Miyamoto, S. Fujinami, Y. Ukaji, Tetrahedron 70 (2014) 6623-6629. DOI:10.1016/j.tet.2014.06.118 |
| [24] |
A. Ghosh, A.K. Pandey, P. Banerjee, J. Org. Chem. 80 (2015) 7235-7242. DOI:10.1021/acs.joc.5b00705 |
| [25] |
B. Wang, M. Liang, J. Tang, et al., Org. Lett. 18 (2016) 4614-4617. DOI:10.1021/acs.orglett.6b02253 |
| [26] |
M. Liang, S. Zhang, J. Jia, et al., Org. Lett. 19 (2017) 2526-2529. DOI:10.1021/acs.orglett.7b00804 |
| [27] |
Y. Liao, B. Zhou, Y. Xia, et al., ACS Catal. 7 (2017) 3934-3939. |
| [28] |
Y. Zhan, T. Liu, J. Ren, Z. Wang, Chem. -Eur. J. 23 (2017) 17862-17866. |
| [29] |
M. Alajarin, D. Banon, A. Egea, et al., Org. Chem. Front. 5 (2018) 2020-2029. DOI:10.1039/C8QO00255J |
| [30] |
Y. Xu, F. Chang, W. Cao, et al., ACS Catal. 8 (2018) 10261-10266. |
| [31] |
R.K. Varshnaya, P. Banerjee, Org. Biomol. Chem. 15 (2017) 5182-5190. DOI:10.1039/c7ob00941k |
| [32] |
S.G. Lee, S. Sin, S. Kim, S.G. Kim, Tetrahedron Lett. 59 (2018) 1480-1483. DOI:10.1016/j.tetlet.2018.03.004 |
| [33] |
S.G. Lee, S.G. Kim, Tetrahedron 74 (2018) 3671-3678. DOI:10.1016/j.tet.2018.12.006 |
| [34] |
R. Talukdar, D.P. Tiwari, A. Saha, M.K. Ghorai, Org. Lett. 16 (2014) 3954-3957. |
 2020, Vol. 31
2020, Vol. 31 




