It is well known that the application of agrochemicals has brought great increase in crop production and numerous benefits for our lifestyles [1, 2]. However, the environmental pollution and resistance problems associated with the conventional pesticides prompt researches to discover and develop more new agro-chemicals with good properties, such as high efficiency, low toxicity, environmental benignity, which is also a permanent project in pesticide chemistry area [3-6].
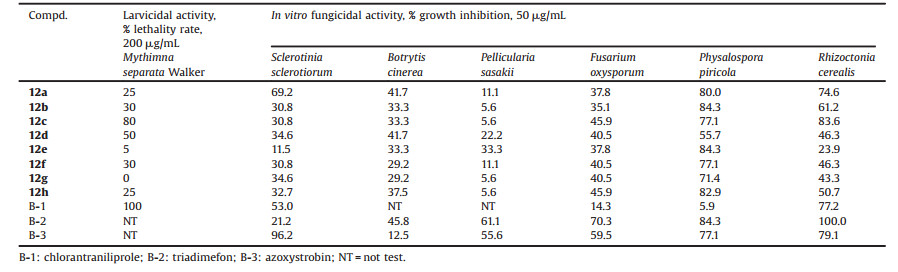
Heterocyclic compounds, an important class of organic matter, have attracted much attention due to their varied interesting structures and diverse applications in a variety of chemical areas, such as pharmacochemistry, pesticide chemistry, material chemis-try [7-9]. Especially, they are indispensable part in almost all kinds of agrochemicals. Biologically important N-pyridylpyrazole deriva-tives are typical heterocyclic compounds, which aroused our intensive attention because of their excellent pesticidal potentials and environmentally friendly properties [10, 11]. For examples, the famous anthranilic diamide insecticides chlorantraniliprole and cyantraniliprole (Fig. 1, I) which have been successfully commercialized by DuPont have such heterocyclic motif [12]; some N-pyridylpyrazole amide derivatives with simple substitu-ents bearing in the benzene ring (Fig. 1, II) were also found to display excellent insecticidal activities [13]; hydrazide [14], β-lactam [15] and benzotriazinone [16] derivatives containing N-pyridylpyrazole group (Fig. 1, III, IV and V), which were synthesized recently via a less concerned amide bridge-modification strategy based on the structures of pyridylpyrazole amide insecticides, were surprisingly found to have remarkable fungicidal activities towards a variety of plant fungi, such as Alternaria solani, Gibberella sanbinetti, Physalospora piricola and Puccinia sorghi Schw. In addition, some N-pyridylpyrazole acylthiourea derivatives (Fig. 1, VI), another kind of amide bridge-modified compounds were also reported exhibit promising insecticidal activities against Mythimna separata Walker, Plutella xylostella, etc. [17]. These results indicated that there still exists great studying potentials for the structural modification of amide bridge part of pyridylpyrazole amides, which inspired us to synthesize more novel heterocyclic compounds with good bio-activities by replacing the amide bridge with other type of groups that may serve as important pharmacophores, such as 1, 2, 4-oxadiazole (e.g., commercial pesticide Tioxazafen) [18].
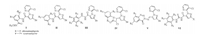
|
Download:
|
| Fig. 1. The structures of some N-pyridylpyrazole compounds with pesticidal activities. | |
The 1, 3-dipolar cycloaddition reaction is one of the most powerful tool for the synthesis of heterocyclic compounds [19]. Among which, 1, 2, 4-oxadiazole is a good example, which can be conveniently constructed using [3 + 2] cycloaddition reaction through imine and dipole intermediate — nitrile oxide [20, 21]. To the best of our knowledge, it has neither yet been involved in the literatures available that a 1, 2, 4-oxadiazole heterocycle was introduced into the structure of pyridylpyrazole derivatives via 1, 3-dipolar cycloaddition reaction, nor the investigation on the pesticidal activities of the corresponding new compounds. With this in mind, a series of novel N-pyridylpyrazole derivatives containing 1, 2, 4-oxadiazole moiety (Fig. 2) were synthesized through 1, 3-dipolar cycloaddition and their structures were studied by means of NMR and single crystal diffraction technologies. The insecticidal and fungicidal activities of the new compounds were explored, and the structure-activity relationships were also analyzed in detail.
|
Download:
|
| Fig. 2. Design of the title compounds 12a-h. | |
According to the reported procedure [22], the intermediate N-hydroxypivalimidoyl chloride (3) was prepared from pivalalde-hyde as starting material via oximation and chlorination, successively (Scheme 1).
|
Download:
|
| Scheme 1. Synthetic route of the intermediate 3. | |
The intermediates 2-amino-5-chloro-N, N-disubstitutedbenza-mide (6a and 6b) were synthesized from 2-amino-5-chlorobenzoic acid 4 referring to a similar procedure in literature (Scheme 2) [23].
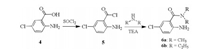
|
Download:
|
| Scheme 2. Synthetic route of the intermediates 6a and 6b. | |
The synthetic route of intermediate pyridylpyrazole aldehyde 10 is shown in Scheme 3, which was prepared from pyridylpyr-azole carboxylic acid (7) as the starting material by esterification, reduction and oxidation reactions, successively [15, 24].
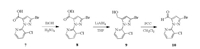
|
Download:
|
| Scheme 3. Synthetic route of the intermediate 10. | |
As shown in Scheme 4, the key intermediate pyridylpyrazole imines 11a-h containing various substituted benzene moieties were synthesized from the condensation of aldehyde 10 (2 mmol) with amines 6 (2.2 mmol), and the reaction can be carried out smoothly in ethanol under acetic acid catalysis.

|
Download:
|
| Scheme 4. Synthetic route of the title compounds 12a-h. | |
Then substituted imines 11a-h (2 mmol) and TEA (2.4 mmol) were dissolved in CH2Cl2 (10 mL), to this solution N-hydroxypiva-limidoyl chloride 3 (2.4 mmol) was added dropwise and the mixture was stirred at 20-40 ℃ for 5 h. Upon completion of the reaction, the solvent was removed under reduced pressure and H2O (20 mL) was added. The aqueous phase was extracted with CH2Cl2 (3×20 mL) and the combined organic phase was washed with brine and dried over Na2SO4. The solvent was removed and the residue was further purified by silica gel column chromatography to give the title compounds 12a-h.
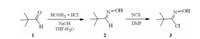
The larvicidal activities of the title compounds 12a-h against oriental armyworm (Mythimna separata Walker) were investigated under the contrast of chlorantraniliprole in a greenhouse, using a general procedure of the leaf-dip method [25]. The in vitro fungicidal activities against Sclerotinia sclerotiorum, Botrytis cinerea, Pellicularia sasakii, Fusarium oxysporum, Physalospora piricola and Rhizoctonia cerealis were tested using the mycelium growth rate method according to reference [26, 27].
For the synthesis of the title 1, 2, 4-oxadiazole compounds 12a-h (Scheme 4), the key intermediate 11a-h can be efficiently prepared from the nucleophilic addition-condensation reaction of the aldehyde and amine intermediates which can be carried out smoothly in ethanol under acetic acid catalysis. However, in the experiment it was found that when using 2-amino-5-chloro-N-substitued-3-methylbenzamide as the arylamine material (e.g. 13, Scheme 5), the expected imine product (14) cannot be obtained smoothly, but the cyclizing compound 15 was formed owing to the existence of the amide moiety (-CONH-) in the adjacent position of amino group. This result is same as that of conducting the reaction in a 4-toluene sulfonic acid/toluene system [28]. The further oxidation of compound 15 with NaOCl gave rise to its dehydrogenation product 16. Treated with triethylamine, the intermediate N-hydroxypivalimidoyl chloride (3) generated 1, 3-dipole nitrile oxide in situ, which further reacted with imine intermediate 11 via a [3 + 2] 1, 3-dipolar cycloaddition reaction to efficiently give the desired 1, 2, 4-oxadiazole compounds 12a-h (yield 53% 74%). The possible mechanism is shown in Scheme 6. When there are two groups at the ortho-positions of amino group (-NH2) of arylamine reactant, e.g. 2, 4, 6-trichlorophenylamine or 2-amino-5-chloro-N, N, 3-trimethylbenzamide, such [3 + 2] cycload-dition reaction was found difficult to occur, which may due to the great affect of steric hindrance from both ortho groups of -NH2 to its reactivity.
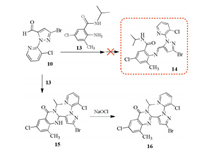
|
Download:
|
| Scheme 5. The reaction of intermediates 10 and 13. | |

|
Download:
|
| Scheme 6. Possible synthetic mechanism for the title compounds 12. | |
Compounds 12a-h were identified by melting point, 1H NMR and 13C NMR spectra. The measured elemental analysis or HRMS data were also consistent with the corresponding calculated values. Due to the presence of one chiral carbon in the 1, 2, 4-oxadiazole part, the NMR spectra of some compound appeared as obvious enantiomers coexistence. When R1 = Cl or CH3, the 1H NMR and 13C NMR spectra for the corresponding compounds 12a-h showed up two sets of characteristic peaks. Interestingly, when R1= CH3, two sets characteristic peaks showed an integral ratio of 1:1 for the corresponding peaks of the same type of proton. Comparatively, when R1 = R2 = H, that is the phenyl substituent connected with oxadiazole ring, no apparent phenomenon of two set peak signals was found. This may due to the steric effect of the substituents in the benzene ring. In 1H NMR, the typical proton of oxadiazole-H was observed at δ 6.66-6.44, lower than that of the pyrazole-H (d 6.89-6.64). In 13C NMR, the chiral carbon signal in the 1, 2, 4-oxadiazole part of compounds 12a-h was observed at δ 93.2-89.3, the carbon signal at δ 110.7-100.0 was derived from the resonance of pyrazole-C(H). Moreover, the proton and quaternary carbon signals of tert-butyl group appeared at δ 1.21-1.04 and 29.5-28.1, respectively.
The structures of compounds 12c (CCDC No.: 1947274) and 12g (CCDC No.: 1947414) were further confirmed by single crystal X-ray diffraction analysis (Fig. 3). The collected diffraction data were provided in Supporting information. From the molecular struc-tures, it can be seen that the torsion angles of N(5)-C(10)-N(4)-O(1) for 1, 2, 4-oxadiazole are 5.883(305)o (12c) and 4.944(286)o (12g), which indicates that the oxadiazole ring skeleton is not coplanar. It also can be found that the absolute configuration of the chiral carbon for the two compounds are S (12c) and R (12g), respectively, which result is in consistency with the possible enantiomers coexistence phenomenon (R- and S- isomers) from NMR evidences. Furthermore, from the Fig. 3, it shows that the pyrazole ring and the benzene ring are located on the opposite of the 1, 2, 4-oxadiazole ring which may be owing to keeping a possible optimal conformation with low energy from steric interaction. The dihedral angle between benzene ring and oxadiazole ring in the cases of 12c and 12g are 83.701(76)o and 75.713(74)o, respectively; and the dihedral angle between pyrazole ring and oxadiazole ring are 83.678(97)o (12c) and 75.938(93)o (12g), respectively. This means both the benzene and pyrazole rings are almost vertical to the oxadiazole ring. Both single crystal structures also show that the substituent R1 in the ortho position of benzene ring (e.g., CH3 and CON(CH3)2) is far from tert-butyl group to keep a possible low energy-favored structure. Therefore, bearing both substituents in two ortho positions of benzene ring would be unfavorable for the stability of the product structure, so would be the [3 + 2] cycloaddition reactivity of the substrate (11). The unsuccessful attempts of the corresponding experiments also reflected such deduction.
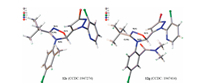
|
Download:
|
| Fig. 3. The single crystal structures of 12c and 12g. | |
As shown in Table 1, most of the title compounds 12a-h exhibited apparent insecticidal activities against oriental army-worm at the concentration of 200 μg/mL. In particular, compounds 12c and 12d possessed lethality rates of 80% and 50%, respectively. It was found that compounds with R1 = CH3 and R2 = Cl or Br showed better insecticidal activity than others. From the fungicidal test results at 50 mg/mL shown in Table 1, we can see that most of compounds displayed weak fungicidal activity towards Pellicularia sasakii. Compound 12a held 69.2% growth inhibition against Sclerotinia sclerotiorum, better than that of chlorantraniliprole (53.0%) and triadimefon (21.2%). Towards Botrytis cinerea, all of the title compounds possessed a higher inhibitory rate (29.2% 41.7%) than that of azoxystrobin (12.5%), but lower than that of triadimefon (45.8%). All the title compounds had moderate activity against Fusarium oxysporum (35.1% 45.9%), better than that of chlorantraniliprole (14.3%). Moreover, most of the compounds showed moderate to excellent fungicidal activity towards Rhizoctonia cerealis with inhibitory rate of 43.3% 83.6%, among which 12c with 83.6% activity is more effective than both chlorantraniliprole (77.2%) and azoxystrobin (79.1%). Noticeably, all the title compounds showed favorable or excellent fungicidal activity against Physalospora piricola, especially 12a, 12b, 12e and 12h whose inhibition activities were 80.0%, 84.3%, 84.3% and 82.9% respectively, were more effective than azoxystrobin (77.1%) and comparable with triadimefon (84.3%). More differently, the control chlorantraniliprole which has the same pyridylpyrazole hetero-cylic structural characteristic had almost no activity towards Physalospora piricola. Through the structure-fungicidal activities analysis, compound 12a (R1 = H, R2 = H) whose fungicidal activities against four fungi (Sclerotinia sclerotiorum, Botrytis cinerea, Physalospora piricola and Rhizoctonia cerealis) were comparatively higher was found to have broader spectrum than others. Towards Physalospora piricola, when R1 is fixed as CH3, the activity sequence corresponding to the R2 is H (12e) > Cl (12c) = CH3 (12f) > Br (12d), which indicates that a smaller group for R2 could help to improve the fungicidal activity of the compound; when R2 = Cl, the R1 bearing an electron-withdrawing group (Cl (12b) or C(=O)N(C2H5)2 (12 h)) is better than the electron-donating group (CH3 (12c)). Interestingly, when R1 is fixed as CH3, the fungicidal activity of the compounds against Rhizoctonia cerealis shows a trend of Cl (12c) > Br (12d) = CH3 (12f) > H (12e) for the substituent R2, indicating an advantage of electron-withdrawing group; when R2 is fixed as Cl, R1 exhibits an opposite influence of electron effect compared with the situation of that against Physalospora piricola, that is, the substituent R1 bearing an electron-donating group CH3 (12c) is much better than electron-withdrawing groups — Cl (12b), C(=O)N (CH3)2 (12g) and C(=O)N(C2H5)2 (12h)). Among all of the title compounds, the inhibitory rate of 12a (R1 = H, R2 = H), 12b (R1 = Cl, R2 = Cl), 12c (R1 = CH3, R2 = Cl) and 12h (R1 = C(=O)N(C2H5)2, R2 = Cl) against the two fungi mentioned above are higher than 50%, which could be considered as fungicidal leading compounds. Moreover, compounds 12c and 12d not only had better insecticidal activity, but also possessed relatively favourable fungicidal activities could be made further structural optimizations for the research and development of new heterocyclic agrochemicals.
|
|
Table 1 Biological activities of the title compounds 12a-h. |
In summary, a series of novel N-pyridylpyrazole derivatives containing 1, 2, 4-oxadiazole moiety 12a-h were efficiently syn-thesized by 1, 3-dipolar cycloaddition. Their structures were identified by melting points, 1H NMR, 13C NMR and elemental analysis or HRMS. The single-crystal structures of 12c and 12g were further analyzed, which revealed the stereochemical and substituent oriental characteristics, and the relevance of the structure and the reaction activity of this type of compounds. The bioassay results showed that several compounds held good insecticidal activity against oriental armyworm (Mythimna separata Walker) at 200 μg/mL, particularly, 12c and 12d had a lethality rate of 80% and 50%, respectively. Some of the compounds exhibited favorable fungicidal activities at 50 μg/mL against Physalospora piricola, Rhizoctonia cereal, Sclerotinia sclerotiorum, etc. Especially, most of the compounds displayed over 70% inhibition against Physalospora piricola. Among this 1, 2, 4-oxadiazole type of derivatives, 12a, 12b, 12c and 12h could be considered as new fungicidal leading compounds for further structural optimization. These discoveries along with the struc-ture-activity relationship analysis in this paper will provide useful guidance for the innovative studies on new pyridylpyrazole derivatives and their applications in agrochemical area.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21772103), Tianjin Natural Science Founda-tion (No. 17JCYBJC19900), and the National Key Research and Development Program of China (No. 2017YFD0200505). We thank teachers Lixia Xiong and Xiao Zhang of the Biological Assay Center, Nankai University, for kind bioassay assistance of compounds.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.10.039.
| [1] |
E.C. Oerke, H.W. Dehne, Crop Prot. 23 (2004) 275-285. DOI:10.1016/j.cropro.2003.10.001 |
| [2] |
X.H. Liu, Y.M. Fang, F. Xie, et al., Pest Manag. Sci. 73 (2017) 1900-1907. DOI:10.1002/ps.4556 |
| [3] |
Q. Hou, Y. Jing, X. Shao, Chin. Chem. Lett. 28 (2017) 1723-1726. DOI:10.1016/j.cclet.2017.05.016 |
| [4] |
Y. Zhang, Y. Feng, Z. Li, et al., Chin. Chem. Lett. 28 (2017) 1228-1231. DOI:10.1016/j.cclet.2017.04.003 |
| [5] |
W. Hidalgo, M. Cano, M. Arbelaez, et al., Pest Manag. Sci. 72 (2016) 796-800. DOI:10.1002/ps.4055 |
| [6] |
F.Y. Li, X.F. Guo, Z.J. Fan, et al., Chin. Chem. Lett. 26 (2015) 1315-1318. DOI:10.1016/j.cclet.2015.05.040 |
| [7] |
Y. Ren, Q. Sun, Z. Yuan, et al., Chin. Chem. Lett. 30 (2019) 1233-1236. DOI:10.1016/j.cclet.2019.03.029 |
| [8] |
Y. Zhang, Y.Z. Zhan, Y. Ma, et al., Chin. Chem. Lett. 29 (2018) 441-446. DOI:10.1016/j.cclet.2017.08.035 |
| [9] |
X. Zhu, W. Jiang, W. Cui, et al., Chin. Chem. Lett. 30 (2019) 1133-1136. DOI:10.1016/j.cclet.2019.02.022 |
| [10] |
G.P. Lahm, T.M. Stevenson, T.P. Selby, et al., Bioorg. Med. Chem. Lett. 17 (2007) 6274-6279. DOI:10.1016/j.bmcl.2007.09.012 |
| [11] |
J. Wu, B.A. Song, D.Y. Hu, et al., Pest Manag. Sci. 68 (2012) 801-810. DOI:10.1002/ps.2329 |
| [12] |
K.A. Hughes, G.P. Lahm, T.P. Selby, et al., Patent, WO 2004067528, 2004.
|
| [13] |
W.L. Dong, J.Y. Xu, L.X. Xiong, et al., Molecules 17 (2012) 10414-10428. DOI:10.3390/molecules170910414 |
| [14] |
T. Yan, S. Yu, P. Liu, et al., Chin. J. Chem. 30 (2012) 919-923. DOI:10.1002/cjoc.201100347 |
| [15] |
B.L. Wang, H.W. Zhu, Z.M. Li, et al., Pest Manag. Sci. 74 (2018) 726-736. DOI:10.1002/ps.4770 |
| [16] |
Y. Zhang, H. Zhu, J. Shang, et al., Chin. J. Org. Chem. 39 (2019) 861-866. DOI:10.6023/cjoc201808033 |
| [17] |
B.L. Wang, H.W. Zhu, Y. Ma, et al., J. Agric. Food Chem. 61 (2013) 5483-5493. DOI:10.1021/jf4012467 |
| [18] |
A.C. Liu, J.L. Feng, X.L. He, et al., Agrochemicals 53 (2014) 561-563. |
| [19] |
J.N. Kim, E.K. Ryu, J. Org. Chem. 57 (1992) 1088-1092. DOI:10.1021/jo00030a011 |
| [20] |
N.L. Chavana, N.H. Naika, S.K. Nayak, et al., Arkivoc 2010 (2010) 248-255. |
| [21] |
K.M. Jiang, U. Luesakul, S.Y. Zhao, et al., Omega 2 (2017) 3123-3134. DOI:10.1021/acsomega.7b00490 |
| [22] |
D.K. Miller, C.A. Bailey, R.E. Sammelson, Synthesis 47 (2015) 2791-2798. DOI:10.1055/s-0034-1378737 |
| [23] |
J. Li, D. Wu, X. Xu, et al., Bioorg. Med. Chem. Lett. 26 (2016) 3064-3066. DOI:10.1016/j.bmcl.2016.05.016 |
| [24] |
J. Xu, W. Dong, L. Xiong, et al., Chin. J. Chem. 27 (2009) 2007-2012. DOI:10.1002/cjoc.200990337 |
| [25] |
B. Wang, H. Wang, H. Liu, et al., Chin. Chem. Lett. 31 (2020) 739-745. DOI:10.1016/j.cclet.2019.07.064 |
| [26] |
B. Wang, Y. Shi, Y. Zhan, et al., Chin. J. Chem. 33 (2015) 1124-1134. DOI:10.1002/cjoc.201500436 |
| [27] |
B.L. Wang, H.W. Zhu, Z.M. Li, et al., Pest Manag. Sci. 75 (2019) 3273-3281. DOI:10.1002/ps.5449 |
| [28] |
Y. Zhou, Q. Feng, F. Di, et al., Bioorg. Med. Chem. 21 (2013) 4968-4975. DOI:10.1016/j.bmc.2013.06.060 |
 2020, Vol. 31
2020, Vol. 31