b National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China;
c Shanghai Institute of Pharmaceutical Industry, Shanghai 201203, China
The genus Hypericum (Guttiferae) comprises about 460 species globally distributed in temperate and subtropical regions [1]. There are 64 species in China, and some of them have antibacterial, anti-inflammatory, and hemostatic efficacy [2]. The Hypericum plants have triggered interests of natural product chemists owing to an array of meroterpenoids they generated. These metabolites not only have diverse and complex structures derived from polyprenylated acylphloroglucinol core with prenyl, geranyl, and acyl groups, but also possess intriguing bioactivities including antidepressant, neuroprotective, anti-inflammatory, antitumor, antibacterial, and antiviral activities [3-6].
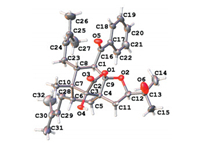
The plant Hypericum patulum is a shrub widespread in China and can be used as a folk medicine for the treatment of gonorrhea, hepatitis, cold, tonsillitis, and bruises [2]. Some novel prenylated acylphloroglucinol meroterpenoids from this plant have been reported previously [7-9]. As a continuing exploration to seek structurally diverse and biologically interesting meroterpenoids from higher plants [10-12], phytochemical investigation on the aerial parts of H. patulum afforded two novel tetracyclic meroterpenoids, hyperinoids A (1) and B (2) (Fig. 1). Both structures with absolute configurations were elucidated by extensive analysis of NMR, MS, and X-ray crystallographic data. Compounds 1 and 2 possess two unprecedented tetracyclic systems of 11, 12-dioxatetracyclo[5.4.3.01, 7.04, 14]tetradecane and 10, 11-dioxatetracyclo[5.3.3.01, 7.04, 13]tridecane, respectively.

|
Download:
|
| Fig. 1. Structures of hyperinoids A (1) and B (2). | |
Nuclear factor-kB (NF-kB) is a key regulator of inflammation, and activation of NF-kB promotes inflammation-associated metabolic disorders such as obesity, type 2 diabetes, and atherosclerosis [13]. Macrophages, as innate immune cells, have been recognized as essential effector cells in the initiation and development of inflammation and insulin resistance [14]. For the discovery of bioactive natural products against inflammation-associated metabolic diseases, compounds 1 and 2 were investi-gated for the inhibitory activities in NF-kB pathway luciferase assay and the effects on the LPS-induced inflammatory responses in macrophages. In this paper, the structural identification and bioactivity evaluation of 1 and 2 are discussed.
Hyperinoid A (1) was isolated as colorless crystals with a specific rotation of 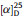
|
|
Table 1 1H (600 MHz) and13C (150 MHz) NMR data of 1 and 2 in CDCl3. |
The planar structure of 1 was established by extensive analysis of its1H-1H COSY and HMBC spectra (Fig. 2A). The1H-1H COSY correlations of H-1/H-8 and H-5/H-6 and the HMBC cross-peaks of H3-10/C-3, C-6, C-8; H2-5/C-3, C-4, C-9; and H-1/C-9 established a cyclopentane (ring A) and a cyclohexane (ring B). The1H-1H COSY correlations of H-11/H-12 and the HMBC networks of H-11/C-4, C-5, C-9, C-13; H-12/C-13; and H3-14, 15/C-12 defined a 3-hydroxy- 2, 2-dimethyltetrahydropyran ring (ring C). The remaining ring (ring D) was formed by a lactone bridge between C-3 and C-9, as suggested by the diagnostic chemical shifts of C-2, C-3, and C-9. Furthermore, the HMBC correlations of H-29/C-6 and H-24/C-8 and the1H-1H COSY correlations of H-6/H2-28/H-29 and H-8/H2-23/H- 24 showed that the two prenyl groups were connected to C-6 and C-8, respectively. The benzoyl group was connected to C-1, as revealed by the HMBC correlations of H-1/C-16 and H-18, H-22/C- 16. Thus, the gross structure of 1 was deduced as a meroterpenoid with an unprecedented 11, 12-dioxatetracyclo[5.4.3.01, 7.04, 14]tetra- decane ring system.

|
Download:
|
| Fig. 2. Key 2D NMR correlations for 1. | |
The coupling constant of H-1/H-8 (J =11.4 Hz) and the H-1/H- 23b ROESY cross-peak (Fig. 2B) indicated that H-1 and H-8 were axially trans-oriented, and they were then assigned an α-orientation and a β-orientation, respectively. The H3-10/H-8 ROESY correlation suggested an equatorial β-orientation for H3-10. The H-1/H-28b, H-1/H-5α, and H-5α/H-12 ROESY correlations revealed that the prenyl group at C-6 and H-12 were α-oriented. The relative configurations at C-3, C-4, and C-9 could not be elucidated by ROESY experiment. But owing to the restriction by the rigid tetradecane ring system, they could be assigned as 3R*, 4S*, and 9R*. A single-crystal X-ray diffraction experiment for 1 was conducted by Ga Kα radiation with an absolute structure parameter of 0.04(6) (Fig. 3). Thus, the absolute configuration of 1 was established as 1S, 3R, 4S, 6S, 7R, 8R, 9R, and 12S.

|
Download:
|
| Fig. 3. Single-crystal X-ray plot for 1. | |
Hyperinoid B (2) was obtained as colorless crystals and had optical activity (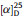

|
Download:
|
| Fig. 4. Key 2D NMR correlations for 2. | |
The relative configurations at C-1, C-6, C-7, and C-8 were consistent with those of 1, as verified by the coupling constant of H-1/H-8 (J =10.8 Hz) and the ROESY correlations of H-1/H-23b, H3-10/H-8, and H-1/H-28b (Fig. 4B). Similar to 1, the 3R*, 4S*, and 9R* configurations were also deduced by the rigidity of the skeleton. The configuration at C-12 could not be defined from the available data. Fortunately, the absolute configuration of 2 was finally assigned as 1S, 3R, 4S, 6S, 7R, 8R, 9R, and 12R by an X-ray crystallographic study (Ga Kα radiation) with an absolute structure parameter of 0.02(9) (Fig. 5). Compound 2 is the first meroterpe-noid possessing a novel 10, 11-dioxatetracyclo[5.3.3.01, 7.04, 13]tride-cane ring system.
|
Download:
|
| Fig. 5. Single-crystal X-ray plot for 2. | |
Compounds 1 and 2 were proposed to biogenetically originate from the prenylated acylphloroglucinol precursor i (Scheme 1). It would undergo intramolecular cyclization to form ii, which after oxidative ring cleavage would give iii. Through decarboxylation, intermediate iv would be formed [7]. After epoxidation and intramolecular nucleophilic addition, the hemiketal intermediate v would be produced. Then, 1 and 2 would be formed via two different ring opening pathways of epoxide.

|
Download:
|
| Scheme 1. Putative biosynthetic pathway for compounds 1 and 2. | |
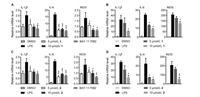
In order to discover natural products against inflammation-associated metabolic diseases, compounds 1 and 2 were evaluated for their anti-inflammatory effects in vitro. Compounds 1 and 2 exhibited significant inhibitory activities in NF-kB pathway luciferase assay with IC50 values of 0.75 0.17 and 1.19 0.48 μmol/L, respectively. In this test, bortezomib (PS-341) was used as the positive control (IC50 = 0.07 0.01 μmol/L). They were further evaluated for influences on the LPS-induced inflammatory responses in RAW 246.7 macrophages and primary mouse BMDM cells. The MTS assay showed no obvious cytotoxicity against RAW 246.7 cells at the tested concentrations (Fig. S1 in Supporting information). The mRNA levels of some pro-inflamma-tory genes, such as IL-1β, IL-6, and iNOS, were downregulated by 1 and 2 (Fig. 6).
|
Download:
|
| Fig. 6. Effects of compounds 1 and 2 on the LPS-induced inflammatory response in macrophages: Effects of 1 (A) and 2 (C) on the LPS-induced genes expression in RAW 246.7 cells. Effects of 1 (B) and 2 (D) on the LPS-induced genes expression in primary mouse BMDM cells. Cells were treated with LPS alone or together with 1 or 2 for 24 h. BAY 11-7082 (10 μmol/L) was used as the positive control. Results are given as mean SEM (n = 3).*P < 0.05,**P < 0.01,***P < 0.001. | |
In summary, hyperinoids A (1) and B (2), two meroterpenoids biogenetically related to prenylated acylphloroglucinols, were obtained from H. patulum. The majority of meroterpenoids from the genus Hypericum belong to the bicyclic polyprenylated acylphloroglucinols featuring a bicyclo[3.3.1]nonane-2, 4, 9-trione core, the adamantane-type with tricyclo[3.3.1.1]decane skeleton, and the homoadamantane-type with tricyclo[4.3.1.1] undecane motif [3]. Different from them, compounds 1 and 2 possess two unprecedented tetracyclic systems of 11, 12-dioxate-tracyclo[5.4.3.01, 7.04, 14]tetradecane and 10, 11-dioxatetracyclo [5.3.3.01, 7.04,13]tridecane, respectively, derived from a rare bicyclo [3.2.1]octane framework [7]. Both of them showed significant anti-inflammatory effects in vitro. They are potent NF-kB pathway inhibitors and can effectively decrease the inflammatory response of macrophages. The novel structures and significant bioactivities of 1 and 2 may provide useful reference for discovering and designing drug leads against inflammation-related metabolic diseases.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis research was financially supported by Fudan-SIMM Joint Research Fund (No. FU-SIMM20181011).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.10.014.
| [1] |
X. Li, N.K.B. Robson, Flora of China, Vol. 13, Science Press, Beijing, 2007, pp. 2-35.
|
| [2] |
State Administration of Traditional Chinese Medicine of the People's Republic of China, Chinese Materia Medica, Vol. 9, Shanghai Scientific and Technical Publishers, Shanghai, 1999, pp. 594-608.
|
| [3] |
X.W. Yang, R.B. Grossman, G. Xu, Chem. Rev. 118 (2018) 3508-3558. DOI:10.1021/acs.chemrev.7b00551 |
| [4] |
H. Bridi, G.C. Meirelles, G.L. von Poser, Phytochemistry 155 (2018) 203-232. DOI:10.1016/j.phytochem.2018.08.002 |
| [5] |
Y.L. Hu, K. Hu, L.M. Kong, et al., Org. Lett. 21 (2019) 1007-1010. DOI:10.1021/acs.orglett.8b04022 |
| [6] |
X.T. Yan, Z. An, Y. Huangfu, et al., Phytochemistry 159 (2019) 65-74. DOI:10.1016/j.phytochem.2018.12.011 |
| [7] |
N. Tanaka, Y. Yano, Y. Tatano, et al., Org. Lett. 18 (2016) 5360-5363. DOI:10.1021/acs.orglett.6b02725 |
| [8] |
Y.Y. Liu, Z. Ao, G.M. Xue, et al., Org. Lett. 20 (2018) 7953-7956. DOI:10.1021/acs.orglett.8b03523 |
| [9] |
Z.N. Wu, Q.W. Niu, Y.B. Zhang, et al., RSC Adv. 9 (2019) 7961-7966. DOI:10.1039/C9RA00277D |
| [10] |
H.B. Liao, C. Lei, L.X. Gao, et al., Org. Lett. 17 (2015) 5040-5043. DOI:10.1021/acs.orglett.5b02515 |
| [11] |
H.B. Liao, G.H. Huang, M.H. Yu, et al., J. Org. Chem. 82 (2017) 1632-1637. DOI:10.1021/acs.joc.6b02800 |
| [12] |
G.H. Huang, Z. Hu, C. Lei, et al., J. Nat. Prod. 81 (2018) 1810-1818. DOI:10.1021/acs.jnatprod.8b00273 |
| [13] |
R.G. Baker, M.S. Hayden, S. Ghosh, Cell Metab. 13 (2011) 11-22. DOI:10.1016/j.cmet.2010.12.008 |
| [14] |
J.C. McNelis, J.M. Olefsky, Immunity 41 (2014) 36-48. DOI:10.1016/j.immuni.2014.05.010 |
 2020, Vol. 31
2020, Vol. 31 


