b College of Chemistry and Chemical Engineering, Lanzhou City University, Lanzhou 730070, China
The extreme toxicity of cyanide (CN-) in physiological systems, as well as the continuing environmental concern caused by its widespread industrial use, has led to considerable research into development of methods for CN- detection [1, 2]. For example, Mergu et al. reported a naphthalimide-benzothiazole conjugate for reaction-based fluorescence detection of CN- [3]. Shiraishi et al. synthesized a coumarin-spiropyran dyad for excellently fluorescent detect CN- [4]. Although some methods have been developed for the ultrasensitive detection of CN- [5-7], unfortunately these methods could not remove target ions. While some other methods could efficiently remove CN- but could not detect CN- [8]. Therefore, developing novel materials for ultrasensitive detection and efficient removal of CN- is a charming subject.
Supramolecular polymers are defined as polymeric systems that extend beyond the molecule [9]. Various dynamical and reversible noncovalent interactions promote monomers to assemble into polymeric structures [10, 11], which makes them not only possess the merits of traditional covalent polymers but also have some unique performances such as stimuli-response properties [12, 13]. Therefore, supramolecular polymers provide a platform for fabricating novel materials with ultrasensitive stimuli responsiveness [14, 15]. In addition, pillar[n]arenes emerged as new supramolecular host macrocycles was first proposed by Ogoshi in 2008 [16]. The special structure of pillararenes could provide various supramolecular interactions such as H-bonding, π-π stacking, metal coordination, etc. [17-19]. These properties endow pillar[n]arenes with opportunities for the construction of stimuli responsive supramolecular polymers [20-25].
Aggregation induced emission (AIE), first coined by Tang in 2001 [26], reveals an important photophysical phenomenon that non-emissive luminogens exhibits enhancement of fluorescent emission at aggregate state [27]. In recent years, new type of supramolecular polymer materials with AIE properties has earned much attention in many fields, including biological areas [28], fluorescent sensors [29], optoelectronic systems [30] and stimuli responses materials [31]. Therefore, the research on AIE provide novel approach for the design of ultrasensitive fluorescent stimuli responsive materials [32, 33].
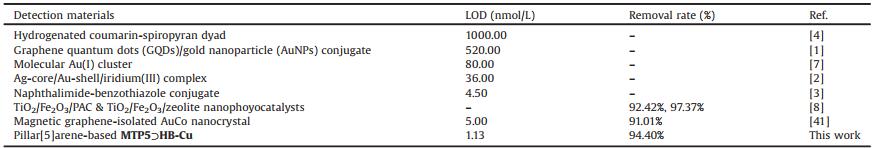
Here, a novel pillar[5]arene-based AIE supramolecular polymer gel MTP5⊃HB was designed and fabricated referring to the research contents of our previous studies [34]. The MTP5⊃HB was constructed by multiple H-bonding and exo-wall π-π stacking interactions between thioacetylhydrazine copillar [5]arene MTP5 and 2, 20-(1, 4-butanediyl)-bis(1H-benzimidazole) HB. Our strategies are demonstrated as follow. First, the thioacetylhydrazine groups on MTP5 provides suitable H-bonding interaction sites and metal coordination sites. Then the HB supplies electron accepting, π-bridging and metal-ions chelating abilities. Impressively, based on this design, the MTP5⊃HB gel is formed and exhibited strong bluish white AIE fluorescence, which can be used to ultrasensitively detect Cu2+ and CN- (Fig. 1). Moreover, the xerogel of in-situ generated metallogel MTP5⊃HB-Cu has excellent capacity to remove CN- in water with 94.40% removal rate.
|
Download:
|
| Fig. 1. Schematic illustration for the formation of MTP5⊃HB and detection mechanism for ions. | |
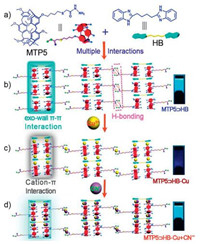
The synthetic route of MTP5 and HB were depicted in Scheme S1 (Supporting information), and they were characterized by 1H NMR, 13C NMR and HRMS (Figs. S1-S6 in Supporting information). Although MTP5 could not self-assemble into a supramolecular gel, stable supramolecular polymer gel MTP5⊃HB was obtained by mixing it with HB in DMSO-H2O binary solvent containing 35% water (Tables S1 and S2 in Supporting information). As shown in Fig. 2a, the sol of MTP5⊃HB had inappreciable fluorescence. However, a stable MTP5⊃HB gel with a remarkable fluorescence enhancement at 468 nm was generated (wt% = 5%, i.e., 50 mg/mL) when the temperature decreased. Simultaneously, a strong bluish white emission was observed under the irradiation of UV–vis lamp at 365 nm. These results indicated the aggregationinduced emission (AIE) property of MTP5⊃HB gel [35].
|
Download:
|
| Fig. 2. (a) Temperature-dependent fluorescence spectra of MTP5⊃HB. (b) Fluorescence spectra of MTP5⊃HB and MTP5⊃HB-M (M is different cation). (c) Fluorescence enhancement by adding various anions into MTP5⊃HB-Cu metallogel. Inset: corresponding photographs under UV lamp irradiation. (d) Fluorescence spectra of MTP5⊃HB-Cu with increasing concentration of CN-. Experiment conditions: wt% = 5%, 35% water fraction in DMSO/H2O, λex = 251 nm. | |
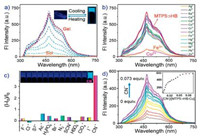
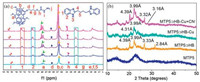
Series experiments were carried out to further investigated the assembly mechanism of the MTP5⊃HB. Firstly, the 1H NMR titration experiment was performed by gradually adding HB into MTP5. Upfield shifts in the signals of protons on HB were observed, while Ha, Hb and Hc located on MTP5 shifted slightly to downfield (Fig. S7 in Supporting information), which suggested that π-electron deficient HB interacted with electron-rich MTP5 via π-π donor-acceptor interaction [36, 37]. Meanwhile, the 2D NOESY NMR spectra exhibited cross peaks at A, B and C (Fig. S8 in Supporting information), which testified that benzimidazole groups on HB were close to the aromatic rings on MTP5. Then, as shown in the concentration-dependent 1H NMR spectrum of MTP5⊃HB (Fig. 3a), both of Ha, Hb, Hc, Hi, Hh on MTP5 and H1, H2, H4 on HB shifted downfield and became broad with increasing the concentration of MTP5⊃HB, indicating the formation of hydrogenbond between MTP5 and HB. Afterwards, the XRD patterns of xerogel MTP5⊃HB showed peaks at 31.47°, 26.75° and 20.21° corresponding to the d-spacing of 2.84 Å, 3.33 Å and 4.39 Å (Fig. 3b), which confirmed that the assembly process of MTP5⊃HB was promoted by the combination of H-bonding, exo-wall π-π stacking and intercolumnar stacking interactions [37-39]. In addition, there was a peak at m/z 1187.5522 in the HRMS spectrum of MTP5⊃HB (Fig. S9 in Supporting information), which could be assigned for [MTP5 + HB + H]+. This result indicated that MTP5 combined with HB in 1:1 stoichiometric ratio. Finally, the SEM morphological features of xerogel MTP5⊃HB showed a regular overlapping laminar morphology (Fig. 4a), which supported the formation of a 3D network structure of MTP5⊃HB.
|
Download:
|
| Fig. 3. (a) Partial concentration-dependent 1H NMR spectra (400 MHz, in DMSO-d6, 298 K) of MTP5⊃HB: (bottom to top) 25.0 mg/mL; 50.0 mg/mL; 75.0 mg/mL; 100.0 mg/mL and 125.0 mg/mL (nMTP5 : nHB = 1 : 1). (b) Corresponding powder X-ray diffraction patterns. Fig. 4. SEM images of (a) xerogel MTP5⊃HB, (b) MTP5⊃HB-Cu, (c) MTP5⊃HB-Cu + CN-. | |
The fluorescence detection property of MTP5⊃HB for cations was studied by diffusing various cations (including Fe3+, Zn2+, Mg2+, Co2+, Zn2+, Hg2+, Ag+, Ca2+, Cu2+, Ni2+, Cr3+, Pb2+, Cd2+ and Ba2+, c = 0.1 mol/L) into MTP5⊃HB, only Cu2+ and Fe3+ ions quenched the fluorescence emission (Fig. 2b). Moreover, the detection limits (LODs) of MTP5⊃HB toward Cu2+ and Fe3+ were determined by fluorescence titration as 1.55 nmol/L and 2.68 nmol/L, respectively (Figs. S10 and S11 in Supporting information), which indicated the sensitivity of MTP5⊃HB were much higher than other reported sensors (Table S3 in Supporting information). After that, the anions recognition performance of in-situ generated metallogel MTP5⊃HB-Cu was investigated by adding different anions such as F-, Cl-, S2-, Ac-, H2PO4-, Br-, N3-, SCN-, HSO4-, ClO4-, I- and CN- (c = 0.1 mol/L) into these metallogel. As shown in Fig. 2C, MTP5⊃HB-Cu could exclusively detect CN-. Based on the fluorescence titration experiment (Fig. 2d), the LOD of MTP5⊃HB-Cu toward CN- was 1.13 nmol/L (Fig. S12 in Supporting information). Compared with other reported sensors, the MTP5⊃HB-Cu possesses higher sensitivity for detecting CN- (Table 1).
|
|
Table 1 Comparison of detection and removal efficiency of MTP5⊃HB-Cu for CN- with other reported sensors. |
The response mechanisms of supramolecular polymer gel towards Cu2+ and CN- were investigated using FT-IR, XRD and SEM. In the FT-IR spectra (Fig. S15 in Supporting information), the stretching vibration absorption peaks of -NH and -C=O transferred from 3427 cm-1 and 1649 cm-1 to 3436 cm-1 and 1627 cm-1, with the addition of Cu2+ into MTP5⊃HB. Meanwhile, new shoulder peaks appeared at 1114 cm-1 and 2999 cm-1, representing the changes of the stretching vibration absorption peaks of C-S-C and =C-H on aromatic ring, respectively. Moreover, in the XRD spectra (Fig. 3b), the peaks at 3.33 Å and 2.84 Å disappeared, but the peak at 4.39 Å had negligible change. These changes indicated that intercolumnar stacking maintained, but H-bonding and exo-wall π-π stacking were destroyed. Additionally, the morphology of xerogel MTP5⊃HB-Cu transformed into fragmentary (Fig. 4b). These results showed that the MTP5⊃HB combined with Cu2+ by coordination interaction with thioacetylhydrazine groups and cation-π interaction.

|
Download:
|
| Fig. 4. SEM images of (a) xerogel MTP5⊃HB, (b) MTP5⊃HB-Cu, (c) MTP5⊃HB-Cu + CN-. | |
Moreover, with the addition of CN- into the MTP5⊃HB-Cu, the stretching vibration absorptions of -NH and -C=O shifted to 3429 cm-1 and 1643 cm-1, respectively, and the peaks at 2999 cm-1 and 1114 cm-1 disappeared (Fig. S15). This consequence can be explained by the strong complexation abilities of CN- with Cu2+, revealing CN- coordinated with Cu2+ in MTP5⊃HB-Cu. Moreover, in the XRD, the peaks around 2u = 26.74° and 28.22° corresponding to the d-spacing of 3.32 Å and 3.16 Å appeared again (Fig. 3b), which verified that CN- coordinated with Cu2+ and the exo-wall π-π stacking generated again. In addition, SEM experiment showed the macromorphology changed from fragmentary into regular overlapped layer structure again after adding CN- into MTP5⊃HB-Cu (Fig. 4C). The above results could be ascribed to the competitive coordination mechanism between CN- and Cu2+.
To explore the application of supramolecular polymer gel MTP5⊃HB-Cu, the removal experiment for CN- were performed. Immersing the xerogel MTP5⊃HB-Cu into CN- solution (c = 3.40 μmol/L) for three hours, the residual content of CN- in water was 0.19 μmol/L [40] (Fig. S13 in Supporting information). The removal rate toward CN- was 94.40%, indicated that MTP5⊃HB-Cu displayed excellent removal capacity toward CN- in aqueous solution (Table 1).Meanwhile, theMTP5⊃HB-Cucouldbe extracted by dichloromethane and reused (Fig. S14 in Supporting information). Portable test kits were prepared for convenient detection of CN- (Fig. S16 in Supporting information). These results are of great significance to detect and remove extreme toxic CN- in water.
In summary, we successfully constructed a supramolecular polymer gel MTP5⊃HB, which emitted bluish white AIE fluorescence and could ultrasensitively detect Cu2+ and Fe3+. Meanwhile, the corresponding metallogel MTP5⊃HB-Cu could be used to continuously detect CN- with high selectivity and ultrasensitivity. More importantly, the xerogel of MTP5⊃HB-Cu showed efficient removal ability for CN- with 94.40% removal rate. Meanwhile, the portable test kit was also prepared for convenient and quick detection of CN-.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21661028, 21662031, 21574104), and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (No. IRT 15R56).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.046.
| [1] |
L.L. Wang, J. Zheng, S. Yang, et al., ACS Appl. Mater. Interfaces 7 (2015) 19509-19515. DOI:10.1021/acsami.5b06352 |
| [2] |
Z.Z. Dong, C. Yang, K. Vellaisamy, et al., ACS Sens. 2 (2017) 1517-1522. DOI:10.1021/acssensors.7b00553 |
| [3] |
N. Mergu, J.H. Moon, H. Kim, et al., Sens. Actuators B:Chem. 273 (2018) 143-152. DOI:10.1016/j.snb.2018.05.165 |
| [4] |
Y. Shiraishi, M. Nakamura, N. Hayashi, et al., Anal. Chem. 88 (2016) 6805-6811. DOI:10.1021/acs.analchem.6b01279 |
| [5] |
E. Jaszczak, Z. Polkowska, S. Narkowicz, et al., Environ. Sci. Pollut. Res. 24 (2017) 15929-15948. DOI:10.1007/s11356-017-9081-7 |
| [6] |
C.F. Chow, P.Y. Ho, W.L. Wong, et al., Chem. -Eur. J. 21 (2015) 12984-12990. DOI:10.1002/chem.201501448 |
| [7] |
C.H. Zong, L.R. Zheng, W.H. He, et al., Anal. Chem. 86 (2014) 1687-1692. DOI:10.1021/ac403480q |
| [8] |
P. Eskandari, M. Farhadian, A.R.S. Nazar, et al., Ind. Eng. Chem. Res. 58 (2019) 2099-2112. DOI:10.1021/acs.iecr.8b05073 |
| [9] |
L. Brunsveld, B.J.B. Folmer, E.W. Meijer, et al., Chem. Rev. 101 (2001) 4071-4098. DOI:10.1021/cr990125q |
| [10] |
H. Li, Y. Yang, F.F. Xu, et al., Chem. Commun. 55 (2019) 271-285. DOI:10.1039/C8CC08085B |
| [11] |
T. Xiao, L. Wang, Chin. Chem. Lett. 29 (2018) 1172-1182. |
| [12] |
C.W. Zhang, B. Ou, S.T. Jiang, et al., Polym. Chem. 9 (2018) 2021-2030. DOI:10.1039/C8PY00226F |
| [13] |
Z.C. Gao, Y.F. Han, S.H. Chen, et al., ACS Macro Lett. 6 (2017) 541-545. DOI:10.1021/acsmacrolett.7b00241 |
| [14] |
Q. Lin, G.F. Gong, Y.Q. Fan, et al., Chem. Commun. 55 (2019) 3247-3250. DOI:10.1039/C8CC09876J |
| [15] |
H.G. Fu, Y. Chen, Y. Liu, ACS Appl. Mater. Interfaces 11 (2019) 16117-16122. DOI:10.1021/acsami.9b04323 |
| [16] |
T. Ogoshi, S. Kanai, S. Fujinami, et al., J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [17] |
C.J. Li, Chem. Commun. 50 (2014) 12420-12433. DOI:10.1039/C4CC03170A |
| [18] |
T. Ogoshi, T. Kakuta, T. Yamagishi, Angew. Chem. Int. Ed. 58 (2019) 2197-2206. DOI:10.1002/anie.201805884 |
| [19] |
P. Li, Y. Cheng, Y. Liu, Chin. Chem. Lett. 30 (2019) 1190-1197. DOI:10.1016/j.cclet.2019.03.035 |
| [20] |
S. Sun, J.B. Shi, Y.P. Dong, et al., Chin. Chem. Lett. 24 (2013) 987-992. DOI:10.1016/j.cclet.2013.07.014 |
| [21] |
Y. Wang, M.Z. Lv, N. Song, et al., Macromolecules 50 (2017) 5759-5766. DOI:10.1021/acs.macromol.7b01010 |
| [22] |
Z.Y. Li, Y.Y. Zhang, C.W. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8577-8589. DOI:10.1021/ja413047r |
| [23] |
T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276. DOI:10.1016/j.cclet.2018.05.039 |
| [24] |
J. Chen, Y. Wang, C. Wang, et al., Chem. Commun. 55 (2019) 6817-6826. DOI:10.1039/C9CC03165K |
| [25] |
S. Sun, D. Lu, Q. Huang, et al., J. Colloid. Interf. Sci. 533 (2019) 42-46. DOI:10.1016/j.jcis.2018.08.051 |
| [26] |
J.D. Luo, Z.L. Xie, J.W.Y. Lam, et al., Chem. Commun. 18 (2001) 1740-1741. |
| [27] |
Y.N. Hong, J.W.Y. Lam, B.Z. Tang, Chem. Soc. Rev. 40 (2011) 5361-5388. DOI:10.1039/c1cs15113d |
| [28] |
D. Ding, K. Li, B. Liu, et al., Acc. Chem. Res. 46 (2013) 2441-2453. DOI:10.1021/ar3003464 |
| [29] |
D.H. Dai, Z. Li, J. Yang, et al., J. Am. Chem. Soc. 141 (2019) 4756-4763. DOI:10.1021/jacs.9b01546 |
| [30] |
J. Huang, N. Sun, P.Y. Chen, et al., Chem. Commun. 50 (2014) 2136-2138. DOI:10.1039/c3cc49313j |
| [31] |
P. Wang, B.C. Liang, D.Y. Xia, Inorg. Chem. 58 (2019) 2252-2256. DOI:10.1021/acs.inorgchem.8b02896 |
| [32] |
A. Pankajakshan, D. Kuznetsov, S. Mandal, Inorg. Chem. 58 (2019) 1377-1381. DOI:10.1021/acs.inorgchem.8b02898 |
| [33] |
L.W. Ma, S. Wang, C.P. Li, et al., Chem. Commun. 54 (2018) 2405-2408. DOI:10.1039/C8CC00213D |
| [34] |
Y.M. Zhang, W. Zhu, W.J. Qu, et al., Chem. Commun. 54 (2018) 4549-4552. DOI:10.1039/C8CC00814K |
| [35] |
J. Mei, N.L.C. Leung, R.T.K. Kwok, et al., Chem. Rev. 115 (2015) 11718-11940. DOI:10.1021/acs.chemrev.5b00263 |
| [36] |
L.Q. Shangguan, H. Xing, J.H. Mondal, et al., Chem. Commun. 53 (2017) 889-892. DOI:10.1039/C6CC08336F |
| [37] |
S.L. Wang, Y.L. Wang, Z.X. Chen, et al., Chem. Commun. 51 (2015) 3434-3437. DOI:10.1039/C4CC08820D |
| [38] |
N. Malviya, M. Das, P. Mandal, et al., Soft Matter 13 (2017) 6243-6249. DOI:10.1039/C7SM01199G |
| [39] |
X.D. Chi, M. Xue, Y. Yao, et al., Org. Lett. 15 (2013) 4722-4725. DOI:10.1021/ol402048n |
| [40] |
S. Nagashima, T. Ozawa, Int. J. Environ. Anal. Chem. 10 (1980) 99-106. |
| [41] |
L.F. Zhang, J.S. Zhang, Z.F. Zheng, et al., Anal. Chem. 91 (2019) 8762-8766. DOI:10.1021/acs.analchem.9b01811 |
 2020, Vol. 31
2020, Vol. 31