b Shanxi Key Laboratory of Green Preparation and Functionalization for Inorganic Materials, Xi'an Key Laboratory of Green Processing for Ceramic materials, School of Material Science and Engineering, Shaanxi University of Science and Technology, Xi'an 710021, China;
c College of Materials&Environmental Engineering, Hangzhou Dianzi University, Hangzhou 310036, China
Li is the most electropositive metal (-3.040 V vs. the standard hydrogen electrode) and has a super high theoretical specific capacity of 3860 mAh/g, and thus, innovative rechargeable battery technology is expected to be achieved through the development of lithium (Li) [1-9]. However, in recent years, the growth of lithium dendrites has repeatedly frustrated the development of recharge-able lithium metal batteries (LiMBs). This uncontrollable phenom-enon severely affects the performance of LiMBs, resulting in low coulombic efficiency, poor cycle stability, and even potential short-circuit safety hazards [10, 11]. To overcome this difficulty and to achieve the commercialization of LiMBs, relevant academic circles and industry have studied new research hotspots and have proposed many strategies for inhibiting lithium dendrite growth [1, 12]. For example, submicron-/nanoporous media [13-16] (e.g., solid electrolyte-modified separators [17-19] and artificial protec-tion layers [20-24]) have been added to LiMBs to inhibit lithium dendrite growth, but the safety, stability, and long cycle life of LiMBs cannot be guaranteed [25].
Recently, researchers have proposed an interface processing method in which the composition of the electrolyte and electrolyte additives are adjusted with the aim of achieving a stable solid electrolyte interface (SEI) film. It has been demonstrated that liquid electrolytes of various lithium salts (such as LiFSI, LiFSI, LiBOB, or their mixtures) [26-28], solvents (such as carbonates, ethers, or ionic liquids) [28] and additives (such as Cs+, LiF, LiNO3 and Li2S8) [29, 30] improve SEI stability. However, most of the methods that have been used to modify SEI film are not sufficiently stable to withstand the effects of mechanical deformation that are caused by repeated Li deposition and peeling [31]. Therefore, on the basis of previous studies, it is particularly important to select an appropriate electrolyte additive to improve the cycle life and cycle stability of the battery.
In this work, we introduced a mixed fiber microporous membrane as the additive to suppress the growth of lithium dendrite. This mixed fiber microporous membrane is generally referred to as a nitric acid-acetic acid mixed cellulose ester microporous membrane. There are many lipophilic groups in the mixed fiber microporous membrane, and it is expected that desired surface doping and grafting of functional groups immobi-lize dissolved electroactive species from the cathode side. Immobilizing these species is helpful for optimizing lithium deposition and inhibiting lithium dendrite growth. A microporous membrane is considered to be a viable alternative lithium metal anode diaphragm in batteries because they can simultaneously reduce lithium dendrites and improve the stability of the coulombic efficiency. Therefore, our purpose is to use the mixed fiber microporous membrane as an electro-chemical active polymer of electrolyte to manipulate the deposition behavior of lithium ions. Upon discharging, lithium ions can react with the mixed fiber microporous membrane and become immobilized. As expected, when the electrolyte was added to the mixed fiber microporous membrane, the overpotential of the symmetric battery was effectively reduced. After 100 cycles, the battery has no lithium dendrites, and the surface is still smooth. The coulombic efficiency is 99% after 500 cycles under a current density of 1 mA/cm2. Herein, the battery with the mixed fiber microporous membrane has a longer cycle life and a higher specific capacity; it also inhibits lithium dendrite growth and improves the coulombic efficiency of lithium.
A coin-type cell (CR2032), diaphragm, and Cu foil were chosen as the parts for constructing the batteries, and they were all from Shenzhen KejingZhida Technology Co., Ltd. The mixed fiber microporous membrane was purchased from Shanghai Compact Film Separation Technology Co., Ltd. Li foil was prepared from Beijing Jinbeinuo Technology Co., Ltd., and the electrolyte was 1 mol/L LiTFSI in DOL/DME (1:1, v/v) with 0.1 mol/L LiNO3.
The mixed fiber microporous membrane was punched into 1.77 cm2 circular disks (i.e., the disks had a 15 mm diameter) and dried at 60 C under vacuum for 24 h. In addition, the mixed fiber microporous membrane and Cu foils or Li foils were put in the coin-type cell; the thin sheets of lithium must be polished. The CR2032-type coin cells (Li|Cu) and symmetric cells (Li|Li) were then constructed in an argon-filled glovebox (< 1 ppm H2O, < 1 ppm O2).
The electrochemical measurements and reaction were con-ducted using the coin-type cell (CR2032) with Li foil as the both counter electrode and reference electrode. Celgard 2400 micropo-rous polypropylene film (thickness of 25 μm, porosity of 6%–46%, and aperture of 0.01–0.1 μm.) was used as the separator for all of the batteries. A battery testing system (LAND CT 2001A, China) was used for the electrochemical measurements and electrochemical plating/stripping of Li.
The Li-ion transference number (tLi+) was determined from a combination of AC impedance and DC polarization (10 mV) measurements with a symmetric Li|electrolyte|Li cell at room temperature. To construct batteries, a membrane filter placed and pressed close to copper, and these Li|Cu cells were galvanostati-cally tested at current densities of 1 mA/cm2 and 2 mA/cm2 using LANHE. Symmetric Li|Li cells were galvanostatically tested at current densities of 1 and 2 mA/cm2 with time-controlled charge and discharge cycles. Electrochemical impedance spectroscopy (EIS) measurements of cells after different numbers of cycles were performed in a frequency ranging from 100 kHz to 100 mHz with an amplitude of 10 mV on a PARSTAT-2273 electrochemical workstation.
The mixed fiber microporous membrane has a pore size of 0.22 mm, a diameter of 50 mm, and a thickness of 100 μm. The mixed fiber microporous membrane material is mainly composed of C, N, and O (Fig. S1 in Supporting information). Also, the mixed fiber microporous membrane is ordinary membrane. The mixed fiber microporous membrane can induce uniform Li+ flux distribution, and this lowers the electrode current density to combat Li dendrite formation and to alleviate changes in the size of Li metal when it undergoes uninterrupted plating/stripping. 1 mL of lithium sulfur electrolyte was added to 5 filter membranes, and 5 mL of lithium sulfur electrolyte was added to 25 filter membranes. The mixed fiber microporous membrane rapidly dissolved in the electrolyte and formed a gel-like substance (a bionic ionic gel electrolyte). Bionic ionic gel electrolytes can rapidly transmit lithium ions and spontaneously form a particle concen-tration layer on the surface of lithium metal anodes. Although the particle concentration layer causes a polarized concentration, the particle concentration layer effectively inhibits lithium dendrite growth and avoids the risk of lithium dendrites puncturing the diaphragms [32].
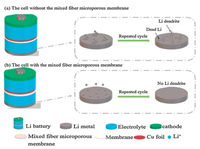
Fig. 1 is a schematic diagram showing that lithium dendrite growth in the lithium anode battery with the mixed fiber microporous membrane is significantly reduced and that there is no dead lithium. This is different from the battery without the mixed fiber microporous membrane. In the battery with the membrane, the structure of the electrolyte remained stable before and after cycling. This method promotes rapid and uniform nucleation of the additive through the surface of the lithium sheet and can inhibit the formation of lithium dendrites.
|
Download:
|
| Fig. 1. Schematic diagram of structural changes in different Li metal anodes. | |
Fig. S2 (Supporting information) shows the evolution of the interface resistance (Ri) of the Li/addition/Li symmetric cell as a function of time. Ri is determined from the size of the semicircle in the high frequency region of a Nyquist plot. The interface resistance should contain contributions from the SEI layer resistance as well as from charge-transfer resistance between an electrode and the electrolyte. The Ri of cells without the additive is 66 Ω cm2, but the Ri of cells with the additive is relatively small with a value of 29 Ω cm2.
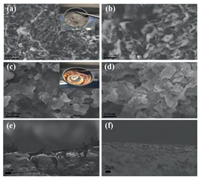
After 100 cycles, the Li metal deposits in the battery remained dissolved in 1, 3-dioxolane for a long time. To corroborate the electrochemical evidence, SEM was used to detect the interfacial structure stability of lithium sheets after plating/peeling multiple times. Based on observations of the plane of the lithium foils (Figs. 2a and b), the cell without the mixed fiber microporous membrane grew many strip shapes that are known to be Li dendrites. However, the surface of the additive-modified electrode (Figs. 2cFigs. 2c and d) does not show any strip shapes. A visual inspection of the disassembled batteries shows a large amount of dead lithium in the battery that does not have the filter film additive, whereas the battery that has the filter film additive has no dead lithium at all. This is because the cell with the mixed fiber microporous membrane suppresses lithium dendrite growth. Fig. 2e shows the surface of the Cu-Li battery that do not have the mixed fiber microporous membrane, and Fig. 2f shows the surface of the Cu-Li battery that has the mixed fiber microporous membrane. It is clear that Fig. 2e shows lithium dendrite growth, whereas Fig. 2f shows no lithium dendrites at all. Thus, adding the mixed fiber microporous membranes has an inhibitory effect on lithium dendrite growth.
|
Download:
|
| Fig. 2. SEM images of the pristine Li anode surface (a, b) and its cross-sectional morphology (e), SEM images of the additive-modified Li anode surface (c, d) and its corresponding cross-sectional morphology (f) after 500 cycles at a current density of 1 mA/cm2 and a capacity of 1 mAh/cm2. | |
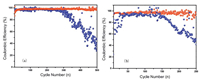
Symmetric cells were cycled with a fixed capacity of 1 mA/cm2 in ether-based electrolytes, and the results are shown in Fig. 3. The stable voltage profile and small hysteresis of the mixed fiber microporous membrane cell are attributed to mitigation of the ion concentration gradient and stabilization of the SEI formation. Cyclic voltammetry, electrochemical impedance spectroscopy, and other test methods were used to examine the electrochemical behavior of the cell that had the mixed fiber microporous membrane. The coulombic efficiency of the cell that did not have the mixed fiber microporous membrane began to decline after 300 cycles when tested at a current density of 1 mA/cm2 (Fig. 3a). However, the cell that had the mixed fiber microporous membrane was always stable through 500 cycles under a current density of 1 mA/cm2. Moreover, for a battery that had the mixed fiber microporous membrane, the coulombic efficiency began to decline after 250 cycles. Also, batteries were attenuated for 150 cycles under a current density of 2 mA/cm2. Thus, the coulombic efficiency of the cells that had the mixed fiber microporous membrane was stable, and the cycle life was improved.
|
Download:
|
| Fig. 3. Coulombic efficiency of Li plating test with the mixed fiber microporous membrane cells (red) and normal cells (blue) at current densities of (a) 1 mA/cm2 (1 mAh/cm2) and (b) 2 mA/cm2 (1 mAh/cm2). | |
The behaviors of molecules were investigated in detail to probe the mechanism of the mixed fiber microporous membrane additive. Li-Cu half cells with pristine electrolyte and with electrolyte that contained the mixed fiber microporous membrane were assembled, and then cyclic voltammetry (CV) analysis was conducted with a cut off voltage window of 0.01–3 V at a scan rate of 0.0001 mV/s; the results are shown in Fig. 4a. In the absence of the mixed fiber microporous membrane, the peak of the CV curve is not obvious, and the fluctuation is not very big, which indicates that the reaction is not very violent. As seen in the CV curves, for electrolyte containing addition, there is one marked cathodic peak at 0.486 V and an anodic peak located at 0.737 V for the initial cycle. In subsequent cycles, only a pair of redox peaks at 0.486 V and 0.737 V dominates the curves.

|
Download:
|
| Fig. 4. (a) Cyclic voltammetry (CV) scans of the Li-Cu half-cell using electrolyte with the mixed fiber microporous membrane at a scan rate of 0.1 mV/s. (b) Cycling performance of the Li|Li cells with different membranes at a current density of 1 mA/cm2. | |
Symmetric cells for Li-Li metal provide a quantitative evalua-tion of the performance of an important platform without the need for decoupling effects. Increasing Li-Li cell voltage vs. time in a current cycle has been reported in the literature, and the change in voltage shape is usually related to the stability of the electrode. Actually, Li dendrite formation and growth are caused by the lithium ion transference number of an electrolyte because it is closely connected to the Li ion concentration gradient at the electrolyte/electrode interface. Lu et al. reported a single-ion Li+ conducting electrolyte with tethered anions, which presented high Li-ion transference number, exhibited a superior ability to induce uniform Li deposits, and increased the cell lifetime of symmetric Li| Li cells 40-fold. Therefore, suppressing Li dendrites is the primary task at present. The mixed fiber microporous membrane was used as a polymer additive to suppress lithium dendrite growth. Fig. 4b shows the voltage profiles of symmetric cells with electrodes that have the mixed fiber microporous membrane and of the bare Li foil counterparts; the voltage profiles are for 1000 cycles at current densities of 1 and 2 mA/cm2 for comparison. For each electrode, the plating/stripping capacity of Li was maintained with a current density of 1 mAh/cm2. At this current density, the symmetric bare Li cell displays a large Li stripping/plating overpotential (> 40 mV) and exhibits an obvious and irregular fluctuation throughout all of the cycles. In contrast, the cell with the mixed fiber microporous membrane shows a much lower overpotential (20 mV) and also achieves very stable cycling after 120 cycles.The voltage profiles of symmetric cells with/without mixed fiber microporous membrane during the 1st, 40th, 80th and 120th cycles can further elucidate the advantages of electrolyte additive The electrode without mixed fiber microporous membrane shows a very large and irregular fluctuation in the voltage profile with evident hysteresis upon repeated Li plating and stripping. In stark contrast, the symmetric cell with mixed fiber microporous membrane shows outstanding cycling stability with quite stable voltage profiles and constantly low hysteresis over 1000 h (Fig. S3 in Supporting information).
The cell anode was tested in a full cell with a pre-delithiated LiFePO4 cathode to evaluate Li utilization in the cell, which is also a key parameter that indicates the performance of Li metal anodes. The constructed full cell exhibited stable coulombic efficiency at 1 C (Fig. S4 in Supporting information), and this indicates that the coulombic efficiency of the cells with the additive in the nano-composite anode can reach about 98%. In contrast, the coulombic efficiency of the full cell without the additive was about 96%.
In summary, using an acidified cellulose ester in cells enhances the interfacial stability of an SEI film. The acidified cellulose ester in the electrolytes is beneficial for promoting uniform lithium deposition and inhibiting lithium dendrite growth. Compared with the performances of corresponding batteries that do not include any addictive, a high average coulombic efficiency (~99%, Li|Cu cell), stable polarization, and superior cycling stability of the symmetric Li|Li cell are achieved when this novel electrolyte additive is used. Thus, the acidified cellulose ester improves the cycling performance, safety, and service life of the battery.
AcknowledgmentsThis work was supported by "the Fundamental Research Funds for the Central Universities" (No. 51772278). M. Zhang special thanks to the supervisor Prof. Yangai Liu for her support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.055.
| [1] |
E.E. Evarts, Nature 526 (2015) S93. DOI:10.1038/526S93a |
| [2] |
D. Lin, Y. Liu, Y. Cui, Nat. Nanotechnol. 12 (2017) 194-206. DOI:10.1038/nnano.2017.16 |
| [3] |
M.D. Tikekar, S. Choudhury, Z.Y. Tu, L.A. Archer, Nat. Energy 1 (2016) 16114. DOI:10.1038/nenergy.2016.114 |
| [4] |
C. Yan, Y.X. Yao, X. Chen, et al., Angew. Chem. Int. Ed. 130 (2018) 14251-14255. DOI:10.1002/ange.201807034 |
| [5] |
J. Cui, T.G. Zhan, K.D. Zhang, D. Chen, Chin. Chem. Lett. 28 (2017) 2171-2179. DOI:10.1016/j.cclet.2017.11.039 |
| [6] |
X. Shen, H. Liu, X.B. Cheng, C. Yan, J.Q. Huang, Energy Storage Mater. 12 (2018) 161-175. DOI:10.1016/j.ensm.2017.12.002 |
| [7] |
T. Yang, Y.G. Liu, D. Yang, et al., Energy Storage Mater. 17 (2019) 374-384. DOI:10.1016/j.ensm.2018.05.024 |
| [8] |
T. Yang, D.X. Yang, Y.G. Liu, et al., Electrochim. Acta 290 (2018) 193-202. DOI:10.1016/j.electacta.2018.08.084 |
| [9] |
T. Yang, D.X. Yang, Q. Mao, et al., Nanotechnology 30 (2019) 155701. DOI:10.1088/1361-6528/aafe42 |
| [10] |
Q. Yun, Y.B. He, W. Lv, et al., Adv. Mater. 28 (2016) 6932-6939. DOI:10.1002/adma.201601409 |
| [11] |
W. Xu, J. Wang, F. Ding, et al., Energy Environ. Sci. 7 (2014) 513-537. DOI:10.1039/C3EE40795K |
| [12] |
K. Xie, Y. You, K. Yuan, et al., Adv. Mater. 29 (2017) 1604724. DOI:10.1002/adma.201604724 |
| [13] |
Y. Lu, Z. Tu, L.A. Archer, Nat. Mater 13 (2014) 961-969. DOI:10.1038/nmat4041 |
| [14] |
K.K. Fu, Y. Gong, J. Dai, et al., PANS 113 (2016) 7094-7099. DOI:10.1073/pnas.1600422113 |
| [15] |
D. Zhou, R. Liu, Y.B. He, et al., Adv. Energy Mater. 6 (2016) 1502214. DOI:10.1002/aenm.201502214 |
| [16] |
X.Q. Zhang, X.B. Cheng, X. Chen, C. Yan, Q. Zhang, Adv. Funct. Mater. 27 (2017) 1605989. DOI:10.1002/adfm.201605989 |
| [17] |
W. Luo, L. Zhou, K. Fu, et al., Nano Lett. 15 (2015) 6149-6154. DOI:10.1021/acs.nanolett.5b02432 |
| [18] |
X.B. Cheng, H.J. Peng, J.Q. Huang, et al., ACS Nano 9 (2015) 6373-6382. DOI:10.1021/acsnano.5b01990 |
| [19] |
P. Bai, J. Li, F.R. Brushett, M.Z. Bazant, Energ. Environ. Sci. 9 (2016) 3221-3229. DOI:10.1039/C6EE01674J |
| [20] |
B. Zhu, Y. Jin, X. Hu, et al., Adv. Mater. 29 (2017) 1603755. DOI:10.1002/adma.201603755 |
| [21] |
C.B. Bucur, A. Lita, N. Osada, J. Muldoon, Energ. Environ. Sci. 9 (2016) 112-116. DOI:10.1039/C5EE03056K |
| [22] |
X.B. Cheng, T.Z. Hou, R. Zhang, et al., Adv. Mater. 28 (2016) 2888-2895. DOI:10.1002/adma.201506124 |
| [23] |
K. Xie, K. Yuan, K. Zhang, et al., ACS Appl. Mater. Inter. 9 (2017) 4605-4613. DOI:10.1021/acsami.6b14039 |
| [24] |
Z. Liang, G. Zheng, C. Liu, et al., Nano Lett. 15 (2015) 2910-2916. DOI:10.1021/nl5046318 |
| [25] |
N. Li, W. Wei, K. Xie, et al., Nano Lett. 18 (2018) 2067-2073. DOI:10.1021/acs.nanolett.8b00183 |
| [26] |
L. Suo, Y.S. Hu, H. Li, M. Armand, L. Chen, Nat. Commun. 4 (2013) 1481. DOI:10.1038/ncomms2513 |
| [27] |
J. Qian, W.A. Henderson, W. Xu, et al., Nat Commun. 6 (2015) 6362. DOI:10.1038/ncomms7362 |
| [28] |
J. Zheng, M.H. Engelhard, D. Mei, et al., Nat. Energy 2 (2017) 17012. DOI:10.1038/nenergy.2017.12 |
| [29] |
F. Ding, W. Xu, G.L. Graff, et al., J. Am. Chem. Soc. 135 (2013) 4450-4456. DOI:10.1021/ja312241y |
| [30] |
W. Li, H. Yao, K. Yan, et al., Nat. Commun. 6 (2015) 7436. DOI:10.1038/ncomms8436 |
| [31] |
G. Zheng, S.W. Lee, Z. Liang, et al., Nat. Nanotechnol. 9 (2014) 618-623. DOI:10.1038/nnano.2014.152 |
| [32] |
Y. Guo, Y. Ouyang, D. Li, et al., Energy Storage Mater. 16 (2019) 203-211. DOI:10.1016/j.ensm.2018.05.012 |
 2020, Vol. 31
2020, Vol. 31 

