b University of Science and Technology of China, Hefei 230026, China;
c Department of Chemistry, Northeast Normal University, Changchun 130021, China;
d State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China;
e Jilin Biomedical Polymers Engineering Laboratory, Changchun 130022, China
Poly(α-amino acid)s, also known as synthetic polypeptides, are a kind of polymer with repeated amino acid units in the backbone, which have been intensively used as biocompatible and biodegradable polymers for various biomedical applications, such as drug/gene delivery, antimicrobial and tissue engineering [1-6]. Owing to the same peptide backbone in polypeptides, the physicalchemical properties (such as solubility, chargeability and secondary structure) and even the biological properties of polypeptides are largely dependent on the side chain structure [6-9]. Thus, it is of great importance to prepare polypeptides with side chain functionalities for desirable biomedical applications.
Reactive oxygen species (ROS), including hydrogen peroxide, superoxide, hydroxyl radical, etc., are natural by-products of cell metabolism, which play important roles in cellular signaling and homeostasis [10, 11]. However, increased generation of ROS can cause damages to lipid, proteins and DNA in cells, which is closely associated with various pathologies, such as inflammation, cancer and cardiovascular diseases [12, 13]. Based on the elevated ROS level, a number of ROS-responsive polymers have been prepared and used for targeted delivery of drugs to diseased tissues or cells [14-18]. To date, ROS-responsive polypeptides are mainly prepared through ring-opening polymerization (ROP) of alkylated derivatives of cysteine and homocysteine N-carboxyanhydride (NCA) monomers, which have one ROS-responsive thioether group in each side chain. For example, Deming et al. prepared glycopolypeptides via ROP of glycosylated L-cysteine NCA monomers and the obtained polymers exhibited an oxidation-triggered helix-to-coil transition in aqueous solution [19]. Similarly, OEGylated L-cysteine and L-homocysteine polypeptides were also synthesized, which displayed multimodal conformation-switching and oxidation/ thermo-dual responsive properties [20, 21]. In addition, Li et al. reported the synthesis of oxidation/thermo-dual responsive polypeptides with OEG side-chains through thiol-ene and thiol-yne reaction mediated post-polymerization modifications [22, 23]. Nevertheless, few efforts have been devoted to the development of ROS-responsive polypeptides for controlled drug release [24, 25].
Here, we report the design and synthesis of a new class of ROS-responsive polypeptide via ROP of 1, 4-dithian-substituted L-glutamate NCA (DTG-NCA) monomer (Scheme 1). By using an amino-terminated methoxy poly(ethylene glycol) (mPEG-NH2) as the macromolecular initiator, an amphiphilic block copolymer, mPEG-b-PDTG, was synthesized, which could self-assemble into micelles in aqueous media. The resultant mPEG-b-PDTG micelles exhibited structural disintegration upon treatment with H2O2 and the oxidation mechanism was further studied by 1H NMR and FT-IR spectrum. Finally, the potential use of mPEG-b-PDTG micelles for ROS-responsive drug release was also evaluated by using Nile Red (NR) as a model drug.

|
Download:
|
| Scheme 1. (A) Synthesis of mPEG-b-PDTG copolymer. (B) Schematic illustration of the self-assembly and intracellular drug release of mPEG-b-PDTG micelles. | |
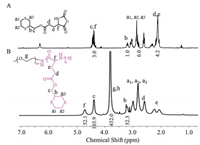
The synthetic route for mPEG-b-PDTG is depicted in Scheme 1. Firstly, the thioether containing molecule, 1, 4-dithian-2-yl-methanol (DTM), was readily synthesized by thiol-yne click reaction between 1, 2-ethanedithiol and propargyl alcohol (Scheme S1 in Supporting information). The corresponding structure was confirmed by 1H NMR and 13C NMR spectrum (Figs. S1 and S2 in Supporting information). The DTM was then conjugated with Boc-Glu-OtBu to form Boc-Glu(ODT)-OtBu (Fig. S3 in Supporting information). Subsequently, the Boc-Glu(ODT)-OtBu was deprotected to obtain DTG (Fig. S4 in Supporting information). DTG-NCA was then synthesized by reaction of DTG with triphosgene in THF [26], and the structure of obtained DTG-NCA was confirmed by 1H NMR spectrum (Fig. 1A). The block copolymer, mPEG-b-PDTG, was synthesized by ROP of DTG-NCA monomer using mPEG-NH2 as the macroinitiator. The resultant mPEG-b-PDTG block copolymer was characterized by 1H NMR spectrum and gel permeation chromatography (GPC). As shown in Fig. 1B, all proton resonance peaks of mPEG-b-PDTG were well assigned, and the degree of polymerization was calculated to be 52, according to the comparison of integral of proton g, h and proton f. In addition, GPC profile showed that the mPEG-b-PDTG had a unimodal peak with Mn =16, 100 g/mol and Ð = 1.71 (Fig. S5 in Supporting information). All these data demonstrated the successful ROP of DTG-NCA and the formation of mPEG-b-PDTG block copolymer.
|
Download:
|
| Fig. 1. Typical 1H NMR spectra of DTG-NCA in CDCl3 (A), and mPEG-b-PDTG in deuterated trifluoroacetic acid (B). | |
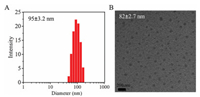
The obtained amphiphilic mPEG-b-PDTG block copolymer can self-assemble into micelles in aqueous solution, consisting of a hydrophobic PDTG core and a hydrophilic PEG outer shell. The critical micelle concentration (CMC) of mPEG-b-PDTG micelles was determined to be 3.3×10-3 mg/mL by using pyrene as the fluorescence probe (Fig. S6 in Supporting information). Fig. 2A shows the results of dynamic light scattering (DLS) measurement of the micelles. The average diameter of the mPEG-b-PDTG micelle was 95±3.2 nm. Moreover, the transmission electron microscopy (TEM) image indicated that the mPEG-b-PDTG micelles had a spherical morphology with a diameter of 82±2.7 nm (Fig. 2B), which was slightly smaller than the diameter value determined by DLS. This should be due to that the micelles were in dehydrated state in TEM image, while the micelles were in hydrated state in DLS measurement.
|
Download:
|
| Fig. 2. DLS measurement (A) and TEM image (B) of the mPEG-b-PDTG micelles. | |
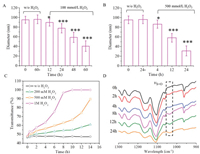
It is well-documented that thioether-containing polymers are typical ROS-responsive materials; and usually, the hydrophobic thioether-containing polymer can be transformed into hydrophilic one via the oxidation conversion of hydrophobic thioether groups into hydrophilic sulfoxide and sulfone groups [27, 28]. Thus, the mPEG-b-PDTG micelles are expected to have ROS-responsiveness. To test it, mPEG-b-PDTG micelles in aqueous solution were treated with or without H2O2, and then the particle sizes were monitored by DLS measurements at different time intervals. As shown in Fig. 3A, when treated with 100 mmol/L H2O2, the size of mPEG-b-PDTG micelles decreased gradually to ~40 nm at 60 h and no valid DLS results could be obtained after 60 h. Moreover, a quicker decrease of sizes was observed when a higher concentration of H2O2 (500 mmol/L) was used (Fig. 3B). In contrast, negligible size variation was observed without treatment with H2O2 during the same period of time (Figs. 3A and B). All these results indicated that the mPEG-b-PDTG micelles were ROS-responsive and the disintegration of mPEG-b-PDTG micelles occurred upon treatment with H2O2. Furthermore, the TEM was applied to confirm the oxidationinduced disintegration of mPEG-b-PDTG micelles. As shown in Fig. S7 (Supporting information), irregular nano-aggregates were observed after incubation with H2O2 for 48 h, indicating the disintegration of micelles upon oxidation by H2O2.
|
Download:
|
| Fig. 3. Size changes of mPEG-b-PDTG micelles in the presence of (A) 100 mmol/L H2O2 and (B) 500 mmol/L H2O2 at 25 ℃. * and *** indicate P < 0.05 and P < 0.001, respectively, compared with the w/o H2O2 group. (C) Plots of transmittance changes of mPEG-b-PDTG micelles solution (2.0 mg/mL) in the presence of different concentrations of H2O2. (D) 900-1300 cm-1 FT IR window of the mPEG-b-PDTG micelles (1.0 mg/mL) after treatment with H2O2 (500 mmol/L) for different times. The intensity of all spectra was normalized against the vas CH2 band at 2926 cm-1. | |
Next, turbidity measurement was also performed to determine the ROS-responsive property of mPEG-b-PDTG micelles and, the results are shown in Fig. 3C. No transmittance changes could be observed in the absent of H2O2, while significant increases of transmittance were observed when treated with H2O2 (Fig. 3C and Fig. S8 in Supporting information). And the increases of transmittance would become quicker and more obvious for the micelles when a higher concentration of H2O2 was used. These results are consistent with the above-mentioned DLS measurements, which again suggest that the mPEG-b-PDTG micelles have excellent ROS-responsiveness.
To deeply understand the oxidation-responsiveness of mPEG-b-PDTG, the oxidation process of mPEG-b-PDTG was firstly characterized by FT-IR at different time intervals. As shown in Fig. 3D, a new peak at 1030 cm-1, corresponding to S=O stretching vibration band, was clearly observed after incubation with H2O2 for 2 h, and becoming increasingly visible at prolonged incubation times. At the same time, no peaks ascribed to sulfones could be detected in FT-IR spectra. These results indicate that the thioethers in mPEG-b-PDTG were oxidatively converted into sulfoxides by H2O2 (Fig. S9 in Supporting information), which is consistent with the previous report [24, 28]. Next, the oxidation-induced structural change of 1, 4-dithiane in the side chain was also investigated by 1H NMR characterization by using DTM as the model molecule. It is clearly observed that the proton resonance signals at 2.63–2.99 ppm (a1, a2 and a3) and 3.61 ppm (c) gradually change into corresponding proton resonance signals at 2.61–3.41 ppm (a'1, a'2 and a'3) and 3.94 ppm (c') (Fig. S10 in Supporting information). The significant changes of chemical shift and peak shape also indicate the oxidation of thioethers into sulfoxides, which is in line with the FT-IR characterizations. Furthermore, the increased Mn of oxidized mPEG-b-PDTG was observed in GPC analysis, which further confirmed the oxidation-induced structural changes in the side chains (Fig. S5). Taken together, these results demonstrate that, upon treatment with H2O2, the thioether groups in the side chains can be oxidized into sulfoxide groups, which would result in the conversion of hydrophobic PDTG segment into hydrophilic polymer and subsequently lead to the disassembly of micelles.
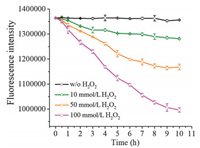
To test the potential use of mPEG-b-PDTG for ROS-responsive drug release, Nile Red (NR), used as a model hydrophobic drug, was loaded into the micelles by a typical dialysis method, and the drug release behavior was monitored in the presence of different concentrations of H2O2. As shown in Fig. 4, there was almost no NR released from mPEG-b-PDTG micelles in the absence of H2O2, indicating the good stability of drug-loaded mPEG-b-PDTG micelles. In comparison, significant decreases of fluorescence, corresponding to the release of NR, were clearly observed in the presence of H2O2, and the higher concentration of H2O2 would result in quicker release of NR. This result should be ascribed to the H2O2-induced disintegration of the micelles that has been previously demonstrated by the DLS and turbidity measurements. In addition, the cellular uptake behavior of NR-loaded mPEG-b-PDTG micelles was further studied by confocal laser scanning microscopy. As shown in Fig. S11 (Supporting information), the NR fluorescence in cells gradually increased over time, indicating the efficient internalization of mPEG-b-PDTG micelles by MCF-7 cells. It has been reported that ROS, such as H2O2, superoxide and hydroxyl radical, are found to be overproduced in many types of pathological tissues, such as cancer and inflammation [10, 11]. Therefore, these preliminary results suggest the promising use of mPEG-b-PDTG as a ROS-responsive drug delivery platform for treatment of cancer and other diseases [29-31].
|
Download:
|
| Fig. 4. The fluorescence intensity of NR-loaded mPEG-b-PDTG micelles monitored at different concentrations of H2O. | |
Finally, the cell cytotoxicity of the obtained mPEG-b-PDTG copolymer in mouse melanoma B16F10 cells and mouse fibroblast L929 cells were tested by MTT assay. As shown in Figs. S12 and S13 (Supporting information), the mPEG-b-PDTG copolymer exhibited negligible cell cytotoxicity toward B16F10 cells or L929 cells (> 90% cell viability), even at a concentration as high as 500 μg/mL. Moreover, the oxidation product of mPEG-b-PDTG copolymer also showed no cytotoxic effects on L929 cells (Fig. S14 in Supporting information). These results suggest a good biocompatibility for the mPEG-b-PDTG copolymer.
In summary, we have successfully developed a new class of polypeptide bearing 1, 4-dithiane pendants for ROS-responsive drug release. An amphiphilic block copolymer, mPEG-b-PDTG, was prepared via ROP of DTG-NCA and was capable to form micellar assemblies in aqueous solution. The mPEG-b-PDTG micelles underwent an oxidation-triggered disassembly due to the oxidation conversion of hydrophobic thioether group into hydrophilic sulfoxide group in the side chains. Then, the ROS-responsive drug release capability of the mPEG-b-PDTG micelles was further demonstrated by using Nile Red as the model drug. In sum, with the facile synthetic process and good biocompatibility, the mPEG-b-PDTG copolymer may be promisingly used as a ROS-responsive drug delivery platform for treatment of cancer and other diseases.
AcknowledgmentsThis work was financially supported by National Key Research and Development Program of China (No. 2016YFC1100701), the National Natural Science Foundation of China (Nos. 51573184, 51520105004 and 51833010) and the Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2017266).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.010.
| [1] |
C.L. He, X.L. Zhuang, Z.H. Tang, H.Y. Tian, X.S. Chen, Adv. Health. Mater. 1 (2012) 48-78. DOI:10.1002/adhm.201100008 |
| [2] |
C. Deng, J. Wu, R. Cheng, et al., Prog. Polym. Sci. 39 (2014) 330-364. DOI:10.1016/j.progpolymsci.2013.10.008 |
| [3] |
H. Lu, J. Wang, Z. Song, et al., Chem. Commun. 50 (2014) 139-155. DOI:10.1039/C3CC46317F |
| [4] |
W. Shen, P. He, C. Xiao, X. Chen, Adv. Health. Mater. 7 (2018) 1800354. DOI:10.1002/adhm.201800354 |
| [5] |
J. Zha, X. Jiang, Chin. Chem. Lett. 29 (2018) 1079-1087. DOI:10.1016/j.cclet.2018.05.026 |
| [6] |
C. Xiao, J. Ding, C. He, X. Chen, Acta Polym. Sin. (2018) 45-55. |
| [7] |
J. Huang, A. Heise, Chem. Soc. Rev. 42 (2013) 7373-7390. DOI:10.1039/c3cs60063g |
| [8] |
T.J. Deming, Chem. Rev. 116 (2016) 786-808. DOI:10.1021/acs.chemrev.5b00292 |
| [9] |
Y. Gao, C. Dong, Chin. Chem. Lett. 29 (2018) 927-930. DOI:10.1016/j.cclet.2017.09.042 |
| [10] |
C.C. Winterbourn, Nat. Chem. Biol. 4 (2008) 278-286. DOI:10.1038/nchembio.85 |
| [11] |
P.D. Ray, B.W. Huang, Y. Tsuji, Cell. Signal. 24 (2012) 981-990. DOI:10.1016/j.cellsig.2012.01.008 |
| [12] |
D. Trachootham, J. Alexandre, P. Huang, Nat. Rev. Drug Discov. 8 (2009) 579-591. DOI:10.1038/nrd2803 |
| [13] |
M. Schieber, Navdeep S. Chandel, Curr. Biol. 24 (2014) R453-R462. DOI:10.1016/j.cub.2014.03.034 |
| [14] |
C.C. Song, F.S. Du, Z.C. Li, J. Mater. Chem. B 2 (2014) 3413-3426. DOI:10.1039/C3TB21725F |
| [15] |
C. Tapeinos, A. Pandit, Adv. Mater. 28 (2016) 5553-5585. DOI:10.1002/adma.201505376 |
| [16] |
Q. Xu, C. He, C. Xiao, X. Chen, Macromol. Biosci. 16 (2016) 635-646. DOI:10.1002/mabi.201500440 |
| [17] |
J. Wang, Y. Zhang, E. Archibong, F.S. Ligler, Z. Gu, Adv. Biosyst. 1 (2017) 1700084. DOI:10.1002/adbi.201700084 |
| [18] |
W. Tao, Z. He, Asian J. Pharm. Sci. 13 (2018) 101-112. DOI:10.1016/j.ajps.2017.11.002 |
| [19] |
J.R. Kramer, T.J. Deming, J. Am. Chem. Soc. 134 (2012) 4112-4115. DOI:10.1021/ja3007484 |
| [20] |
X. Fu, Y. Shen, W. Fu, Z. Li, Macromolecules. 46 (2013) 3753-3760. DOI:10.1021/ma400678w |
| [21] |
J.R. Kramer, T.J. Deming, J. Am. Chem. Soc. 136 (2014) 5547-5550. DOI:10.1021/ja500372u |
| [22] |
X. Fu, Y. Ma, J. Sun, Z. Li, RSC Adv. 6 (2016) 70243-70250. DOI:10.1039/C6RA17427B |
| [23] |
X.H. Fu, Y.N. Ma, J. Sun, Z.B. Li, Chinese J. Polym. Sci. 34 (2016) 1436-1447. DOI:10.1007/s10118-016-1861-x |
| [24] |
Q. Xu, C. He, K. Ren, C. Xiao, X. Chen, Adv. Health. Mater. 5 (2016) 1979-1990. DOI:10.1002/adhm.201600292 |
| [25] |
S. Yu, C. Wang, J. Yu, et al., Adv. Mater. 30 (2018) 1801527. DOI:10.1002/adma.201801527 |
| [26] |
C. Xiao, C. Zhao, P. He, et al., Macromol. Rapid Comm. 31 (2010) 991-997. DOI:10.1002/marc.200900821 |
| [27] |
C. Xiao, J. Ding, L. Ma, et al., Polym. Chem. 6 (2015) 738-747. DOI:10.1039/C4PY01156B |
| [28] |
P. Carampin, E. Lallana, J. Laliturai, et al., Macromol. Chem. Phys. 213 (2012) 2052-2061. DOI:10.1002/macp.201200264 |
| [29] |
C. Wang, H. Sang, Y. Wang, et al., Nano Lett. 18 (2018) 7045-7051. DOI:10.1021/acs.nanolett.8b03015 |
| [30] |
X. Li, Y. Zhang, Z. Ma, et al., Chin. Chem. Lett. 30 (2019) 489-493. DOI:10.1016/j.cclet.2018.03.019 |
| [31] |
S. Gao, G. Tang, D. Hua, et al., J. Mater. Chem. B 7 (2019) 709-729. DOI:10.1039/C8TB02491J |
 2020, Vol. 31
2020, Vol. 31 

