b Institute of Clinical Pharmacy and Pharmacology, Jining First People's Hospital, Jining Medical University, Jining 272000, China;
c Yidu Central Hospital of Weifang, Weifang 261000, China
Biothiols including glutathione (GSH), cysteine (L-Cys), and homocysteine (Hcy) play significant roles in maintaining many physiological processes [1-3]. Abnormal levels of these biothiols in vivo can also serve as indicators of many diseases. For example, L-Cys deficiency is associated with liver damage, slow growth in children, and impaired antioxidant defenses [4, 5]. Hence, it is of great meaning to monitor their intracellular concentrations. Many techniques have been used to detect biothiols such as high-performance liquid chromatography (HPLC), UV–vis spectroscopy, mass spectroscopy, and electrochemical methods. However, these methods usually pose certain of limitations such as long time consuming, high experimental costs, and complex sample processing. Several novel detection methods have been developed in recent years. Among them, fluorescence detection is a convenient method due to its high sensitivity, simplicity, low cost, and great potential for the intracellular bioimaging [6-8]. Most existing fluorescent probes for biothiols are small organic molecules. However, these probes often suffer some disadvantages since they are tended to quench or aggregate in water [9-12], which restricts their use in vivo. Nevertheless, these probes are mainly reaction-based, so they are susceptible to the surrounding environment and suffer a long response time [9, 12-14]. Nanomaterials or a nanoplatform and metal-organic framework (MOF) are potential candidates to solve these issues [4, 15-19]. Gold nanoparticles (AuNPs) are ultra-efficient fluorescence quenchers that show the highest quenching efficiency (up to 99%) through fluorescence resonance energy transfer (FRET) [20-22]. This feature of AuNPs combined with their biocompatibility, small size, and easy modifiability has made AuNP-based conjugates attractive for use as platforms for various bioanalytes [23-26].
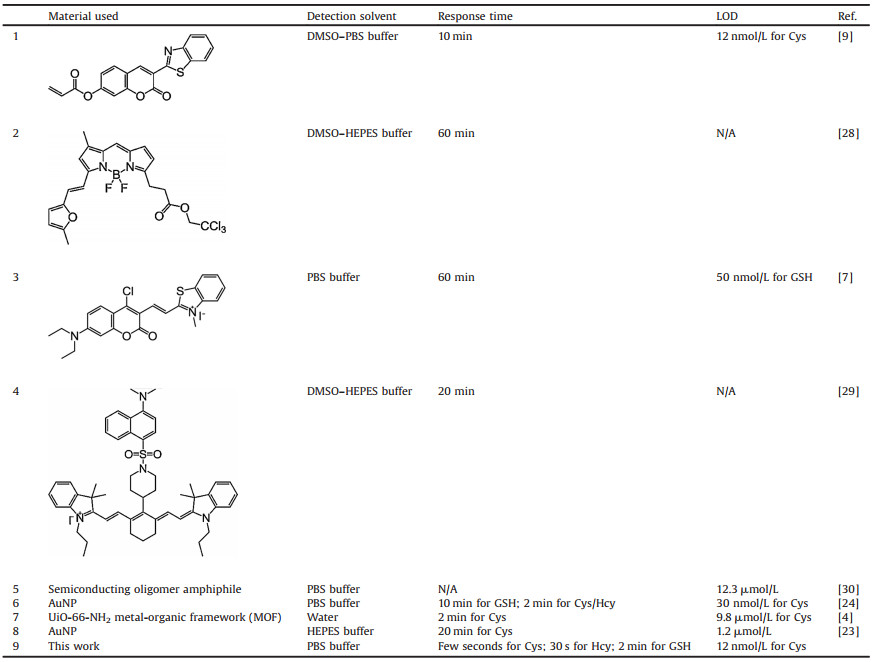
In this paper, we constructed a water-soluble nanosensor (named AuNP-PN) based on the FRET between AuNPs and the organic fluorophore (2-butyl-6-pyridin-4-yl-benzo[de]isoquinoline-1, 3-dione, named PN) which could rapidly sense to the biothiols. In our strategy, PN served as a fluorophore and AuNPs performed as efficient fluorescence quenchers. As illustrated in Scheme 1, upon the biothiol addition, PN molecules were liberated from the AuNPs surface through ligand-exchange displacement based on the strong affinity of Au for the thiol groups, resulting in a dramatic increase in fluorescence.
|
Download:
|
| Scheme 1. Schematic representation of biothiols induced turn-on fluorescence of the sensor. | |
The synthesis process of PN is shown in the Scheme S1 (Supporting information). Synthesis of compound 1: 4-Bromo-1, 8- naphthalic anhydride (0.415 g, 1.5 mmol) and butylamine (0.163 mL, 1.65 mmol) were added into ethanol (10 mL) at room temperature, and refluxed at 90 ℃ for 4 h. The reaction mixture was cooled to room temperature, filtered and washed with a small amount of ethanol. The solvent was evaporated under reduced pressure, and the crude product was purified by column chromatography (petroleum ether:ethyl acetate = 7:1) to afford compound 1 as a light yellow powder (335.1 mg, 69%). Synthesis of PN: Compound 1 (111.7 mg, 0.34 mmol) was stirred with boric acid (161.6 mg, 0.4 mmol) in THF (10 mL). Tetraphenylphosphine palladium (25 mg) and 50% K2CO3 solution (1.5 g) were added and the reaction mixture was refluxed at 80 ℃ under N2 protection for 8 h. The reaction mixture was extracted with CH2Cl2 for three times. The organic phase was collected and dried over MgSO4, then evaporated under reduced pressure, and the crude product was purified by column chromatography (petroleum ether:ethyl acetate = 7:1) to afford PN as light yellow powder (107 mg, 65%). 1H NMR (400 MHz, DMSO-d6, TMS): δ 0.93 (t, 3H, J = 8.0 Hz), 1.35 (d, 2H, J = 8.8 Hz), 1.63 (m, 2H, J = 8.0 Hz), 4.03 (t, 2H, J = 7.6 Hz), 7.62 (m, 2H, J = 1.6 Hz), 7.8 (d, 2H, J = 10.8 Hz), 8.21 (dd, 1H, J = 8. 0 Hz), 8.80 (m, 2H, J = 8.0 Hz) (Fig. S1 in Supporting information).
AuNPs were synthesized by the addition of HAuCl4 (10 mg) to a refluxing, rapidly stirred solution of sodium citrate (20 mg) in water (100 mL, pH 7.4). The solution was refluxed for an additional 15 min. A purple color appeared, indicating the formation of gold nanoparticles. PN (5 μL, 5 mmol/L ethanol solution) was added into 3 mL of AuNPs solution in the dark. The resulting mixture was stirred for 10 min at room temperature. The obtained AuNP-PN solution was diluted with 10 mmol/L PBS buffer solution to get the stock solution.
The cell experiments were conducted in Hepatic stellate cells. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, HyClone) on a 96-well plate for 12 h, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin antibiotic (100 U of penicillin and 100 μg/mL of strep tomycin sulfate) at 37 ℃ in a humidified incubator containing 5% CO2. For the CCK-8 cell viability assay, cells in a 96-well plate were incubated with AuNP-PN solution for 24 h. After that, the supernatant was removed and the cells were washed with phosphate buffered saline (PBS) solution. Then, 100 μL DMEM and 10 μL CCK-8 stock solutions (5 mg/mL in PBS, pH 7.4) were added and incubated for 4 h at 37 ℃. The optical density (OD) of the mixture was measured at 450 nm and the cell viability was estimated according to the following equation (Eq. 1).
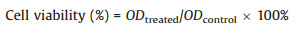
|
(1) |
where ODcontrol is the OD obtained in the absence of AuNP-PN and ODtreated is the OD obtained in the presence of AuNP-PN.
Experiments of sensing ability of AuNP-PN for biothiols were performed in 0.1 mol/L PBS. Hepatic stellate cells were incubated with 100 μL AuNP-PN for 30 min at 37 ℃. For the control experiment, Hepatic stellate cells were pretreated with NEM (0.5 mmol/L) and incubated with AuNP-PN for 30 min at 37 ℃. The culture medium was removed and washed three times with 0.1 mol/L PBS before observation.
As described above, AuNPs were prepared in presence of trisodium citrate by reduction of HAuCl4 as previously described [27] and then modified with PN through a place-exchange reaction of pyridine groups with citrate protectors. AuNP-PN was constructed via the interaction between PN and AuNPs. The PN core was used as the fluorescence donor, and the pyridine groups acted as binding sites with AuNPs though the N atom. As shown in Fig. S2 (Supporting information), there is overlap among the emission wavelength of PN and the absorbance wavelength of AuNPs, which ensured a strong FRET effect. The slight red-shift in the absorbance spectra of the AuNP-PN solution compared with the AuNP solution indicated successful binding between PN and AuNPs. The obtained AuNP-PN composite is red with no fluorescence emission, and the particle diameter is 22 nm (Fig. S3 in Supporting information).
Experiments to evaluate the sensing ability of AuNP-PN to biothiols were carried out. As shown in Fig. 1A, the AuNP-PN solution emitted no fluorescence, but dramatic fluorescence at 420 nm and a brightblue-colorwere clearlyobservedon additionofbiothiols (Hcy, GSH, L-Cys, 5 μmol/L). The mechanism is illustrated in Scheme 1. In the absence of thiols, the fluorescent chromophore of PN was coordinated to AuNPs through weak N-Au interactions, which quenched the fluorescence due to FRET. The recoveryof fluorescence is likely attributable from the thiol-triggered place-exchange reaction resulting the free PN fluorophore.

|
Download:
|
| Fig. 1. (A) Photographic image (inset) and emission spectra of AuNP-PN response to biothiol. The concentration of the biothiols was 5 μmol/L. (B) Real-time emission spectra of the emission spectra by adding various thiols: Cys, Hcys and GSH. The concentration of the biothiols was 2 μmol/L. All measurements were acquired at 25 ℃ in 10 mmol/L PBS, pH 7.4, with emission at 420 nm. | |
The AuNP-PN was constructed by adding PN to AuNP solution. To ensure sensor sensitivity, we optimized the amount of AuNP solution to the critical point where PN fluorescence was totally quenched and the biothiol response was the most sensitive. As shown in Fig. S4 (Supporting information), the fluorescence intensity of PN decreased when the amount of AuNP solution increased from 0 mL to 3 mL. Fluorescence was totally quenched when the amount of AuNPs was 3 mL, suggesting that this was the optimal amount of AuNP solution.
The response time of AuNP-PN composite to biothiols was studied subsequently. When the biothiol was added into the sensing system, AuNP-PN reacted with L-Cys immediately, but with Hcy and GSH was completed within 30 s and ~2 min, respectively (Fig. 1B). To the best of our knowledge, this is the fastest detection method for biothiols to date (Table 1) [4, 7, 9, 23, 24, 28-30]. The ultra-fast response time can overcome the limitations of organic fluorescent probes, making it suitable for real-time biothiols detection which holds great promises for serving as POC testing devices. The rapid response time was mainly due to the stronger coordination ability of the thiols with AuNPs compared with that of the pyridyl moiety of PN. Appropriate modification of the binding site may further shorten the response time. In addition, the high water-solubility significantly enhanced the place-exchange reaction. The different response times of the three biothiols are mainly due to steric effects, especially for GSH.
|
|
Table 1 An overview on recently reported probes of biothiols and their main characteristic. |
The effect of pH on the response of AuNP-PN to biothiols was also investigated (Fig. S5 in Supporting information). In the absence of thiols, AuNP-PN was stable and showed almost no fluorescence in the pH range of 4.0-9.0. It exhibited weak fluorescence in the pH range of 9.0–11.0 due to protonation of the N atom in PN and its release from the AuNP surface. Upon the addition of GSH, AuNP-PN emitted strong and stable fluorescence at 420 nm in the pH range of 6–10, indicating that the sensor can be stably used in neutral and weakly basic conditions (pH 6–9), which covers the physiological pH range and enables use in cells or tissue.
Under the optimized conditions, we performed fluorescence titration experiments with Hcy. The fluorescence intensity of AuNP-PN increased along with the Hcy concentrations (Fig. 2). The limit of detection (LOD) was calculated as 12 nmol/L in the concentration range of 0–10 mmol/L (Eq. 2).

|
(2) |
|
Download:
|
| Fig. 2. (A) Fluorescence spectrum of AuNP-PN in the presence of different concentrations of Hcy (0–10 μmol/L). (B) Fluorescence intensity of AuNP-PN versus Hcy concentration. All measurements were acquired at 25 ℃, pH 7.4, with emission at 420 nm. | |
where s is the standard deviation of blank measurements, and k is the slope of the linear equation [31].
The normal intracellular level of Hcy is 5–10 μmol/L [32]. Therefore, the sensor is sufficiently sensitive for Hcy in living cells.
To evaluate the selectivity, other amino acids were used to challenge the sensing method. As shown in Fig. S6 (Supporting information), only biothiols induced fluorescence; no obvious changes took place when other amino acids were added, even at a 10-fold higher concentration, indicating a highly selective sensing ability of AuNP-PN.
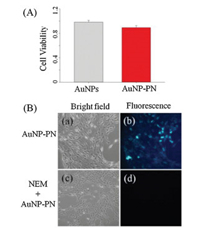
Prior to further biological applications, AuNP and AuNP-PN cytotoxicity was evaluated with CCK-8 assays. As shown in Fig. 3A, the viability of hepatic stellate cells remained above 85% after exposure. The results demonstrate that AuNP-PN shows excellent biocompatibility
|
Download:
|
| Fig. 3. (A) Cell viability of Hepatic stellate cells exposed to AuNP-PN and AuNP over a period of 24 h treatment. (B) Microscopy imaging of living Hepatic stellate cells: (a and b) The cells incubated with AuNP-PN for 30 min; (c and d) The cells pretreated with NEM. | |
To demonstrate the practical utility of the sensor in biological samples, intracellular biothiol imaging was performed in living cells. As shown in Fig. 3B, after hepatic stellate cells were incubated with AuNP-PN at 37 ℃ for 30 min, intracellular green fluorescence was observed (Fig. 3B–b). In contrast, no signal was observed when hepatic stellate cells were pretreated with N-ethylmaleimide (NEM) [11, 29], a known thiol trapping reagent (Fig. 3B–d), indicating that the strong fluorescence originated from intracellular biothiols. These findings demonstrate that the sensor has great promise to image and detect biothiols in living cells.
Though the sensor has been successfully used for imaging biothiols in living cells, limitations do exist to restrict its further use. The relative short emission wavelength of the sensor has poor penetration ability and is easily influenced by ground UV absorption and fluorescence of physiological media like blood and serum. Near infrared (NIR)-Ⅱ fluorescence has stronger penetration ability and may be a promising method to overcome this limitation [33]. We are currently trying to synthesize NIR-Ⅱ fluorescence fluorophores and nanomaterials.
In conclusion, an ultra-fast fluorescent sensor for biothiols was developed based on the FRETeffect between the fluorophore PN and AuNPs, which effectively quenched the fluorescence of the fluorophore. In the presence of biothiols, PN were liberated from the AuNP surface and showed a turn-on fluorescence. The highly selective sensor has appreciable water solubility, an ultra-fast response time (a few seconds for L-Cys), and a low detection limit (12 nmol/L for Hcy). Moreover, the sensor is not cytotoxic and has been successfully used to image biothiols in hepatic stellate cells. Collectively, these findings indicate that AuNP-conjugates may offer a potential platform for biothiol sensing both in vitro and in vivo.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors gratefully appreciate the support from the National Natural Science Foundation of China (Nos. 81971678 and 81671756), Key Research Project of Science and Technology Foundation of Hunan Province (Nos. 2017SK2093, 2018GK5004 and 2019SK2211), and Projects of Medical and Health Technology Development Program in Shandong Province (No. 2018WS471).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.10.005.
| [1] |
T.V. Mishanina, M. Libiad, R. Banerjee, Nat. Chem. Biol. 11 (2015) 457-464. DOI:10.1038/nchembio.1834 |
| [2] |
D.M. Townsend, K.D. Tew, H. Tapiero, Biomed. Pharmacother. 57 (2003) 145-155. DOI:10.1016/S0753-3322(03)00043-X |
| [3] |
T. Finkel, N.J. Holbrook, Nature 408 (2000) 239-247. DOI:10.1038/35041687 |
| [4] |
S. Sharma, S.K. Ghosh, ACS Omega 3 (2018) 254-258. DOI:10.1021/acsomega.7b01891 |
| [5] |
S. Shahrokhian, Anal. Chem. 73 (2001) 5972-5978. DOI:10.1021/ac010541m |
| [6] |
T. Ueno, T. Nagano, Nat. Methods 8 (2011) 642-645. DOI:10.1038/nmeth.1663 |
| [7] |
J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577. DOI:10.1021/ja409578w |
| [8] |
J. Ge, R. Cai, L. Yang, et al., ACS Sustain. Chem. Eng. 6 (2018) 16555-16562. DOI:10.1021/acssuschemeng.8b03684 |
| [9] |
Q. Zhang, D. Yu, S. Ding, G. Feng, Chem. Commun. 50 (2014) 14002-14005. DOI:10.1039/C4CC04978K |
| [10] |
L. He, X. Yang, K. Xu, Y. Yang, W. Lin, Chem. Commun. 53 (2017) 13168-13171. DOI:10.1039/C7CC07296A |
| [11] |
L. Zhai, Z. Shi, Y. Tu, S. Pu, Dyes Pigments 165 (2019) 164-171. DOI:10.1016/j.dyepig.2019.02.010 |
| [12] |
F. Yu, X. Han, L. Chen, Chem. Commun. 50 (2014) 12234-12249. DOI:10.1039/C4CC03312D |
| [13] |
S.Y. Lim, K.H. Hong, D.I. Kim, H. Kwon, H.J. Kim, J. Am. Chem. Soc. 136 (2014) 7018-7025. DOI:10.1021/ja500962u |
| [14] |
P. Xing, Y. Shi, Q. Li, et al., Talanta 179 (2017) 326-330. |
| [15] |
Y. Wen, H. Dong, K. Wang, Y. Li, Y. Li, ACS Appl. Mater. Interfaces 10 (2018) 11457-11466. DOI:10.1021/acsami.7b19201 |
| [16] |
S. Wuttke, A. Zimpel, T. Bein, et al., Adv. Healthc. Mater. 6 (2017) 1600818. DOI:10.1002/adhm.201600818 |
| [17] |
M. Markina, N. Stozhko, V. Krylov, M. Vidrevich, K. Brainina, Talanta 165 (2017) 563-569. DOI:10.1016/j.talanta.2017.01.012 |
| [18] |
J. Liu, H. Qiu, Chin. Chem. Lett. 30 (2019) 1545-1546. DOI:10.1016/j.cclet.2019.07.045 |
| [19] |
Y. Li, X. Wang, C. Xing, et al., Chin. Chem. Lett. 30 (2019) 1440-1444. DOI:10.1016/j.cclet.2019.03.014 |
| [20] |
M. Swierczewska, S. Lee, X. Chen, Phys. Chem. Chem. Phys. 13 (2011) 9929-9941. DOI:10.1039/c0cp02967j |
| [21] |
G. Yue, S. Su, N. Li, et al., Coord. Chem. Rev. 311 (2016) 75-84. DOI:10.1016/j.ccr.2015.11.009 |
| [22] |
W. Lan, Q. Tan, J. Qiao, G. Shen, L. Qi, Chin. Chem. Lett. 30 (2019) 1627-1630. DOI:10.1016/j.cclet.2019.05.019 |
| [23] |
S.H. Lo, M.C. Wu, S.P. Wu, Sensor. Actuat. B:Chem. 221 (2015) 1366-1371. DOI:10.1016/j.snb.2015.08.015 |
| [24] |
J. Xu, H. Yu, Y. Hu, M. Chen, S. Shao, Biosens. Bioelectron. 75 (2016) 1-7. DOI:10.1016/j.bios.2015.08.007 |
| [25] |
H. Zhang, X. Liu, M. Liu, et al., Biosens. Bioelectron. 99 (2018) 625-636. DOI:10.1016/j.bios.2017.08.033 |
| [26] |
S.Q. Chai, W.Y. Lv, J.H. He, et al., Anal. Chem. 91 (2019) 6761-6768. |
| [27] |
S.K. Ghosh, A. Pal, S. Kundu, S. Nath, T. Pal, Chem. Phys. Lett. 395 (2004) 366-372. DOI:10.1016/j.cplett.2004.08.016 |
| [28] |
D. Zhai, S.C. Lee, S.W. Yun, Y.T. Chang, Chem. Commun. 49 (2013) 7207-7209. DOI:10.1039/c3cc43480j |
| [29] |
J. Yin, Y. Kwon, D. Kim, et al., J. Am. Chem. Soc. 136 (2014) 5351-5358. DOI:10.1021/ja412628z |
| [30] |
C. Xie, Y. Lyu, X. Zhen, Q. Miao, K. Pu, ACS Appl. Bio. Mater. 1 (2018) 1147-1153. DOI:10.1021/acsabm.8b00353 |
| [31] |
M. Zhu, M. Yuan, X. Liu, et al., Org. Lett. 10 (2008) 1481-1484. DOI:10.1021/ol800197t |
| [32] |
Y. Yue, F. Huo, X. Li, et al., Org. Lett. 19 (2016) 82-85. |
| [33] |
S. He, J. Song, J. Qu, Z. Cheng, Chem. Soc. Rev. 47 (2018) 4258-4278. DOI:10.1039/C8CS00234G |
 2020, Vol. 31
2020, Vol. 31