b Tianzhu Hongfu Lithium Industry Technology Development Company Limited, Wuwei 733200, China;
c Department of Applied Chemistry, Faculty of Engineering, Kyushu Institute of Technology, Kitakyushu 804-8550, Japan;
d Jiangsu Yangnong Chemical Group Company Limited, Yangzhou 225009, China
In chemical industrial production, there are a variety of reactions involving the element-transfer processes. For example, many oxidation reactions involve the oxygen-transfer process, in which, transferring oxygen from oxidant into substrate is the neat result. In order to achieve a clean and efficient reaction for industrial applications, molecular oxygen as the cheap, abundant and safe oxygen source is preferable, while catalysts are designed and prepared to promote the reactions and they are the keys to achieve the highly efficient oxygen-transfer objectives. Our group has pay much attention on the green reactions with high atom economy [1-5], most of which involve the processes transferring elements such as fluorine, oxygen, hydrogen and even selenium. They are very practical and some have already been successfully applied in real industrial production. Herein, we wish to review the reactions and make a prospect for the developing trend in the field.
2. Fluorine-transfer reactionFluorine-containing compounds are comprehensively employed for their distinctive chemical properties caused by the strong electronegativity of the fluorine element [6, 7]. In this area, LiPF6 as a major lithium electrolyte used in lithium battery and as the intermediate for producing the downstream lithium salts is abundantly consumed and has a continuously growing market for the rapid development of lithium battery industry [8-10]. The tradition method to synthesize this compound employs HF as the fluorine source, which is corrosive and hazardous to the environments, and the operators usually suffer very high security risks [11]. Therefore, developing non-HF method to produce LiPF6 is meaningful for both of the environment-protection and security concerns.
For many years, people have tried to transfer the fluorine from fluoride salts as the alternatives of HF to produce LiPF6. NaF, KF, NH4F and even the relatively expensive LiF have been tested, but all resulted in the failures of low product yield or high production cost. During our continuous investigations on green synthesis, we unexpectedly found that the calcium compounds are active and may be beneficial to enhance the reaction selectivity owing to their low solubilities allowing the slow and sustained release of efficacy to ensure the mild reaction conditions prohibiting the side reactions [12-14]. Reviewing the literatures showed that the distinctive reactivities of calcium compounds have already drawn much attention and are widely used in many reactions to construct a series of complex but useful organic skeletons [3]. The recent progresses in calcium chemistry inspired us designing the synthetic process using CaF2 as the fluorinating agent to produce LiPF6 (Scheme 1).

|
Download:
|
| Scheme 1. Synthesis of LiPF6 using CaF2 as fluorinating agent. | |
In the process, the reaction of PCl5 with CaF2 initially generates PF5. The strong C–F binding energy endows this step very high energy releasing, and this is a very strong driving force facilitating the transfer of fluorine from CaF2 to PF5, which, as a gas, soon leaves the system to push forward the conversion. The subsequent addition reaction of the generated PF5 with LiF in another reaction kettle leads to the LiPF6 product smoothly at room temperature. Transferring fluoro from CaF2 is a meaningful process for the lowcost and safe fluorinating agent. The generated by-product CaCl2 can be sold as snowmelt agent to avoid accumulation. Thus, this synthetic route can utilize the atoms with 100% ratio theoretically to meet the requirement of industrial production. Recently, it has been successfully applied in real industrial production by the Tianzhu Hongfu Lithium Industry Technology Development Company Limited (Hongfu for short) in 2019 [15].
3. Oxygen-transfer reactionThe oxygen-transfer processes are widely involved in oxidation reactions. Compared with other ordinary oxidation reactions [16-18], the characteristic of the oxygen-transfer processes is the transferring of oxygen from the oxidants into the desired products [19, 20]. In our project, selenium-containing compounds/materials were used as catalysts to transfer the oxygen from oxidants to the products because of the excellent oxygen carrier features of selenium. The Se-O bond is weaker than S-O, and this allows the transfer of oxygen from the high valent selenium catalytic species to the product to restart the catalysis cycle. The selenium-catalyzed reactions have already been widely employed in producing high-value-added fine chemicals and the transformations are of industrial application potential for the green processes [21] and the metabolizable features [22] of selenium making this element even safer to the environments than transition metals. Moreover, since China is rich in selenium resources, the related selenium-containing compounds/materials can be obtained at relatively low prices to reduce the catalyst cost in industrial production (the price of Se powder with 99.99% purity is only ca. $70/kg presently).
The diselenide-catalyzed epoxidation reaction of cyclohexene involves a typical oxygen-transfer procedure [23-25]. In the reaction, the catalytic diselenides (1) are first oxidized by H2O2 to produce the seleninoperoxoic acids (2) and this is the step transferring oxygen to the catalytic Se species from the oxidant (Scheme 2). The oxidation of cyclohexene (3) by 2 leads to epoxides (4) and the seleninic acids (5), completing the transfer of oxygen to cyclohexene from the high-valent Se species (Scheme 2). The hydration of 4 leads to the final product trans-1, 2-cyclohexanediol, while the oxidation reaction of 5 regenerates the high-valent Se species 2 to restart the catalysis cycle. Similar oxygen-transfer processes via selenium catalysis are involved in a variety of oxidation reactions, such as the epoxidation reaction of β-ionone [26, 27], the oxidative cracking reaction of alkenes [28-30], the Baeyer-Villiger oxidations [31, 32], and the oxidative ringexpansion of methylenecyclopropanes [33]. It is even employed to produce the polyanilines via the oxidative polymerization reaction [34].
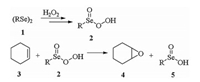
|
Download:
|
| Scheme 2. Oxygen-transfer from H2O2 to cyclohexene. | |
The selenium-catalyzed oxidative deoximation reaction involves another type of oxygen-transfer process [35]. In the processes, the reaction of oximes (6) with seleninic acids (5) directly leads to the products 7 and the nitrogen-containing intermediates 8 (Scheme 3). Different from the single oxygentransfer processes, this step is regarded as an exchange of the oxygen of high-valent Se species with the NOH group of oximes. Decomposition of 8 affords the selenols (9) and nitrosyl hydride (10) (Scheme 3). The oxidation of 9 into high-valent selenium species restarts the catalysis cycle, while the intermediate 10 can be finally oxidized into nitrate, as having been attested by the X-ray photoelectron spectroscopy (XPS) analysis [36]. The addition of ion salt can facilitate the transfer of oxygen from air, so that the reaction can be performed under even milder and safer aerobic conditions free of the explosive H2O2 oxidant [37].

|
Download:
|
| Scheme 3. Oxygen-transfer in Se-catalyzed oxidative deoximation reactions. | |
4. Hydrogen-transfer reaction
Although hydrogenation with gaseous H2 is a highly efficient process in industrial production [38-40], reduction with the active hydrogen species being transferred from small molecules is also a practical process in many cases and it may be even safer than the direct hydrogenation reaction with flammable and explosive H2 gas. In our recent project converting the market-excess acetone into methyl isobutyl ketone (MIBK), isopropanol (IPA) was used as the hydrogen source to reduce the intermediate mesityl oxide (MO). In the process illustrated by Scheme 4, the condensation reaction of acetone (11) in the presence of bases or proline catalysts first led to the intermediate MO (12) [41-43], which was then reduced by IPA (13) to generate MIBK (14) and acetone as a by-product. Pt/C catalyst was used to transfer the hydrogen from 13 and the grabbed hydrogen species on Pt/C was highly active and could reduce 12 into 14. Because acetone can be recycled and reused to produce 12, the neat result of the whole process is considered to be the condensation of 13 with 11, and water is produced as the only by-product. Since all of the catalyst and co-catalyst, the excess solvent and the by-product are recyclable and can be reused in the next turn of reaction, this protocol is very practical. It has been performed in semi-industrial scale production by Jiangsu Yangnong Chemical Group Company Limited (Yangnong for short) [44, 45].

|
Download:
|
| Scheme 4. Hydrogen-transfer from IPA to reduce MO and prepare MIBK. | |
Hydroquinone (HQ) is another significant industrial intermediate being abundantly and extensively used in the production of electrode materials, black-and-white developing agents, anthraquinone and azo dyes, rubber antioxidants, stabilizers, etc. Its price is continuously increasing in recent years for the huge market supply and demand gap. Producing HQ via the hydrogenation reaction of p-benzoquinone (BQ) is a feasible protocol because the starting material BQ can be easily obtained through the selective oxidation reaction of phenol [46]. In a Yangnong technology illustrated in Scheme 5, cyclohexanone (16) is used as the unique hydrogen source to reduce BQ (15) in the presence of Pt/C catalyst [47]. The hydrogen of 16 is initially grabbed by the Pt/C catalyst and then reduces 15 to produce HQ (17). Because the generated by-product cyclohexenone (18) is also a useful intermediate for herbicide production, this method fully utilizes the atoms theoretically and is very practical from the industrial viewpoint. Further investigations on industrializing this technology are still ongoing in the research institute of the company.
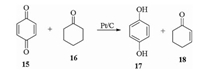
|
Download:
|
| Scheme 5. Hydrogen-transfer from cyclohexanone to p-benzoquinone. | |
5. Selenium-transfer reaction
Organoselenium chemistry has attracted much attention for the unique bio- and chemical-activities of the selenium-containing compounds, which have been widely employed in many fields including the recently booming organoselenium catalysis [48-52]. Thus, synthesisof these compounds is animportant subjectwithgreat industrial application potential. The tradition method to synthesize organoselenium compounds usually includes a selenium-insertion reaction of the Grignard reagents with selenium powder, which is an efficient selenium-transfer reaction with good atom economy. However, the subsequent acidification step leads to the toxic and stinky selenol intermediates and makes this protocol quite environment-unfriendly. Therefore, developing novel seleniumtransfer techniques free of the selenol generation is meaningful for environment-protection.
Recently, we found that, by heating carboxylic acids (19) with selenium powder in N, N-dimethylformamide (DMF) in the presence of N, N-diisopropylethylamine base, the selenization reaction occurred to produce the corresponding selenides (20) and diselenides (21). Evolution of CO2 during the decarboxylation reaction provides a strong driving force for the transformation, but the reaction selectivities are poor and the generated selenide and diselenide products cannot be separated because of their similar polarities. Therefore, this protocol is not preferable to prepare selenides or diselenides for organic synthesis purpose. However, because both of the selenium-containing products are good oxygen carriers and may catalyze the oxidation reactions, their mixtures can be directly used as the catalysts for the related reactions, such as the oxidative deoximation reaction and the Baeyer-Villiger oxidation of the α, β-unsaturated ketones to produce vinyl esters, showing sufficient application potential of the organoselenium catalyst preparation method via the selenol-free decarboxylation couplings with selenium (Scheme 6) [53].

|
Download:
|
| Scheme 6. Selenium-transfer via the decarboxylative coupling of carboxylic acid with selenium powder. | |
Moreover, Na2SO3 can be used as a good selenium carrier for the selenium-transfer processes as well [54]. Heating Na2SO3 with selenium powder in water first leads to Na2SeSO3, in which the selenium element exists as the highly nucleophilic Se2- species. The generated Na2SeSO3 can be used in situ without separation and heating this selenium-containing intermediate with halogenated hydrocarbons (22) in air affords the diselenides (23) in moderate to good yields (Scheme 7). Although mechanism analysis indicates that selenols are generated as the reaction intermediate during the process, they are rapidly oxidized into the stable and inodorous diselenides under the aerobic reaction conditions, making this method very environment-friendly. In comparison with the decarboxylative coupling reactions with selenium powder discussed in the above paragraph, this reaction leads to better product selectivity and diselenides are obtained as the major products.
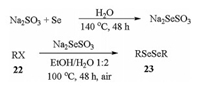
|
Download:
|
| Scheme 7. Selenium-transfer using Na2SO3 as the carrier. | |
6. Photo-driven element-transfer reaction
The energies of our world, including the fossil energy, the wind energy, the hydroenergy, the bioenergy and even the nuclear power all come from the fixed star. Among the above energies, light is the most direct and mild energy from the sun, and using light as the driven force for industrial production is one of the most efficient method for solar energy utilization. Thus, in the field of chemical reaction investigations, photo-driven reactions attract comprehensive attentions during the past decade. In line with the calls for environment-protection, the direct transfer of elements driven by light (especially the visible light) is highly practical and it may be the terminally mature technologies for such kind of reactions.
Very recently, in our group, the visible light-driven oxygen transfer technology has been successfully applied in β-ionone epoxidation being promoted by the photo catalyst of polymeric carbon nitride (PCN), which is a kind of 2D carbon materials prepared from the cheap and abundant nitrogen-containing starting materials such as urea and melamine. In the reaction, water was used as the oxygen source and the photocatalytic water splitting in the presence of PCN led to H2O2, which, catalyzed by the doped selenium in the material, could oxidize the endocyclic C=C bond of β-ionone (24) to produce the useful epoxide 25 (Scheme 8) [55]. The whole process can be seen as an oxygen transfer from water to the substrate, while H2 gas is generated and can be collected and used as green energy or reductant in industry.
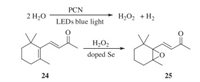
|
Download:
|
| Scheme 8. Photocatalytic oxygen-transfer from water for β-ionone epoxidation. | |
Similarly, the photocatalytic hydrogen-transfer reaction can be achieved by using the water splitting protocol. In our cases, deuterium water (D2O) is used as the deuterium source to synthesize the deuterium-containing compounds, which are high-value-added fine chemicals and may be applied in the deuterium drugs development for pharmaceutical industry. Recently, the PCN-supported palladium catalyst (Pd/PCN) was designed, prepared and used to achieve the above objective. In the presence of PCN, the photocatalytic D2O-splitting produces D2, which, catalyzed by doped palladium in the material, can react with alkenes (26) or alkynes (27) to give the related deuteriumcontaining alkanes 28 and 29 (Scheme 9) [56].
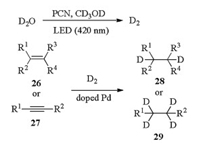
|
Download:
|
| Scheme 9. Photocatalytic deuterium-transfer from D2O to produce deuteriumcontaining compounds. | |
7. Conclusion
A variety of reactions can be concluded as the transfer of certain element. In these reactions, the element source and catalyst are the keys that determine the practicality of the process from the industrial viewpoint. Energy inputs, including the chemical energy, the thermal energy and the light energy, provide the driving force to push forward the element-transfer progresses. Chemical energy comes from the strong binding energy between certain elements, and this only exists in a few examples. Thermal energy is the traditionally employed energy to drive the reaction, but suffers from the poor reaction selectivity and low energy utilization ratio in many cases. New energies, such as the light energy and electric energy, may provide powerful driving force to transfer the elements efficiently and precisely, and are of profound industrial application potential. Transferring a variety of elements (such as F, O, H, Se, Cl) more efficiently and precisely benefits the modern chemical production and is the development trend in the field. This subject deserves further investigations, which are ongoing in our laboratory.
Author contributionsThis review summarizes our staged results on the subjects of the non-HF synthesis of LiPF6 and the green redox reactions to produce fine chemicals hold by Hongfu and Yangnong respectively. Chao Chen, Yitao Cao, Xixi Wu, Yuanli Cai, Jian Liu, Lin Xu and Kehong Ding are the major co-workers in Yangzhou University, Hongfu and Yangnong participating the related projects. They performed the experiments, searched the literatures, performed market investigations and supposed new ideas for the projects and provided assistances in finishing this review. Lei Yu wrote the review and submitted it to the journal.
Declaration of competing interestThe authors declare that they have no conflict of interest.
AcknowledgmentsThis work was financially supported by the Natural Science Foundation of Jiangsu Province (No. BK20181449), Jiangsu Provincial Six Talent Peaks Project (No. XCL-090), and Priority Academic Program Development of Jiangsu Higher Education Institutions.
| [1] |
Y.H. Zheng, A.Q. Wu, Y.Y. Ke, et al., Chin. Chem. Lett. 30 (2019) 937-941. DOI:10.1016/j.cclet.2019.01.012 |
| [2] |
L. Yu, Chem. Regents 41 (2019) 545-549. |
| [3] |
L. Yu, R.R. Qian, X. Deng, et al., Sci. Bull. (Beijing) 63 (2018) 1010-1016. DOI:10.1016/j.scib.2018.06.002 |
| [4] |
H.E. Cao, B.R. Zhu, Y.F. Yang, et al., Chin. J. Catal. 39 (2018) 899-907. DOI:10.1016/S1872-2067(18)63050-5 |
| [5] |
L. Yu, J. Wang, H.E. Cao, et al., Chin. J. Org. Chem. 34 (2014) 1986-1991. DOI:10.6023/cjoc201405004 |
| [6] |
G.K. Liu, X. Li, W.B. Qin, et al., Chin. Chem. Lett. 30 (2019) 1515-1518. DOI:10.1016/j.cclet.2019.03.036 |
| [7] |
D. Zhang, M. Sha, R.M. Pan, et al., Chin. Chem. Lett. 30 (2019) 566-568. DOI:10.1016/j.cclet.2018.11.014 |
| [8] |
M. Yan, W.P. Wang, Y.X. Yin, et al., EnergyChem 1 (2019) 100002. DOI:10.1016/j.enchem.2019.100002 |
| [9] |
Y. Zheng, S.S. Zheng, H.G. Xue, et al., J. Mater. Chem. A 7 (2019) 3469-3491. DOI:10.1039/C8TA11075A |
| [10] |
M.B. Zheng, H. Tang, Q. Hu, et al., Adv. Funct. Mater. 28 (2018) 1707500. DOI:10.1002/adfm.201707500 |
| [11] |
A.A. Smagin, V.A. Matyukha, V.P. Korobtsev, J. Power Sources 68 (1997) 326-327. DOI:10.1016/S0378-7753(97)02652-9 |
| [12] |
H. Zhang, M.T. Han, C.G. Yang, et al., Chin. Chem. Lett. 30 (2019) 263-265. DOI:10.1016/j.cclet.2018.01.035 |
| [13] |
L. Yu, M.T. Han, J. Luan, et al., Sci. Rep. 6 (2016) 30432. DOI:10.1038/srep30432 |
| [14] |
L. Yu, Y.L. Wu, H.E. Cao, et al., Green Chem. 16 (2014) 287-293. DOI:10.1039/C3GC41562G |
| [15] |
J. Liu, Y.L. Cai, C.Q. Xiao, et al., Ind. Eng. Chem. Res. 58 (2019) 20491-20494. DOI:10.1021/acs.iecr.9b04958 |
| [16] |
L.H. Lu, S.J. Zhou, W.B. He, et al., Org. Biomol. Chem. 16 (2018) 9064-9068. DOI:10.1039/C8OB02368A |
| [17] |
K.J. Liu, X.L. Zeng, Y. Zhang, et al., Synthesis 50 (2018) 4637-4644. DOI:10.1055/s-0037-1610231 |
| [18] |
K.J. Liu, S. Jiang, L.H. Lu, et al., Green Chem. 20 (2018) 3038-3043. DOI:10.1039/C8GC00223A |
| [19] |
K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308. DOI:10.1016/j.cclet.2019.10.031 |
| [20] |
K.J. Liu, Y.L. Fu, L.Y. Xie, et al., ACS Sustainable Chem. Eng. 6 (2018) 4916-4921. DOI:10.1021/acssuschemeng.7b04400 |
| [21] |
S. Santoro, J.B. Azeredo, V. Nascimento, et al., RSC Adv. 4 (2014) 31521-31535. DOI:10.1039/C4RA04493B |
| [22] |
M. Rayman, Lancet 379 (2012) 1256-1268. DOI:10.1016/S0140-6736(11)61452-9 |
| [23] |
L. Yu, J. Wang, T. Chen, et al., Chin. J. Org. Chem. 33 (2013) 1096-1099. DOI:10.6023/cjoc2012012049 |
| [24] |
L. Yu, J. Wang, T. Chen, et al., Appl. Organomet. Chem. 28 (2014) 652-656. DOI:10.1002/aoc.3175 |
| [25] |
Y.G. Wang, L.H. Yu, B.C. Zhu, et al., J. Mater. Chem. A 4 (2016) 10828-10833. DOI:10.1039/C6TA02566H |
| [26] |
Y.F. Yang, X. Fan, H.E. Cao, et al., Catal. Sci. Technol. 8 (2018) 5017-5023. DOI:10.1039/C8CY01413B |
| [27] |
L. Yu, Z.B. Bai, X. Zhang, et al., Catal. Sci. Technol. 6 (2016) 1804-1809. DOI:10.1039/C5CY01395J |
| [28] |
C. Liu, J.F. Mao, X. Zhang, et al., Catal. Commun. 133 (2020) 105828. DOI:10.1016/j.catcom.2019.105828 |
| [29] |
T.T. Wang, X.B. Jing, C. Chen, et al., J. Org. Chem. 82 (2017) 9342-9349. DOI:10.1021/acs.joc.7b01245 |
| [30] |
L. Yu, H.E. Cao, X. Zhang, et al., Sustain. Energ. Fuels 4 (2020) 730-736. DOI:10.1039/C9SE00850K |
| [31] |
L. Yu, J.Q. Ye, X. Zhang, et al., Catal. Sci. Technol. 5 (2015) 4830-4838. DOI:10.1039/C5CY01030F |
| [32] |
X. Zhang, J.Q. Ye, L. Yu, et al., Adv. Synth. Catal. 357 (2015) 955-960. DOI:10.1002/adsc.201400957 |
| [33] |
L. Yu, F.L. Chen, Y.H. Ding, ChemCatChem 8 (2016) 1033-1037. DOI:10.1002/cctc.201501309 |
| [34] |
G. Gao, J. Han, L. Yu, et al., Synlett 30 (2019) 1703-1707. DOI:10.1055/s-0037-1612088 |
| [35] |
X.B. Jing, D.D. Yuan, L. Yu, Adv. Synth. Catal. 359 (2017) 1194-1201. DOI:10.1002/adsc.201601353 |
| [36] |
X. Deng, H.E. Cao, C. Chen, et al., Sci. Bull. (Beijing) 64 (2019) 1280-1284. DOI:10.1016/j.scib.2019.07.007 |
| [37] |
C. Chen, X. Zhang, H.E. Cao, et al., Adv. Synth. Catal. 361 (2019) 603-610. DOI:10.1002/adsc.201801163 |
| [38] |
S.P. Wang, S.H. Hou, C. Wu, et al., Chin. Chem. Lett. 30 (2019) 398-402. DOI:10.1016/j.cclet.2018.06.021 |
| [39] |
Y.F. Yang, M.X. Li, H.E. Cao, et al., Mol. Catal 474 (2019) 110450. DOI:10.1016/j.mcat.2019.110450 |
| [40] |
Y.S. Ma, L.Y. Zhang, W. Shi, et al., Chin. Chem. Lett. 30 (2019) 183-186. DOI:10.1016/j.cclet.2018.04.034 |
| [41] |
L. Xu, J.J. Huang, Y.B. Liu, et al., RSC Adv. 5 (2015) 42178-42185. DOI:10.1039/C5RA05741H |
| [42] |
L. Xu, F. Wang, J.J. Huang, et al., Tetrahedron 72 (2016) 4076-4080. DOI:10.1016/j.tet.2016.05.039 |
| [43] |
L. Xu, J.J. Huang, M. Zhang, et al., ChemistrySelect 1 (2016) 1933-1937. DOI:10.1002/slct.201600404 |
| [44] |
F. Wang, L. Xu, J.J. Huang, et al., Mol. Catal. 432 (2017) 99-103. DOI:10.1016/j.mcat.2017.02.010 |
| [45] |
L. Xu, F. Wang, J.J. Huang, et al., Chin. J. Org. Chem. 36 (2016) 2232-2235. DOI:10.6023/cjoc201603044 |
| [46] |
F. Wang, L. Xu, C. Sun, et al., Chin. J. Org. Chem. 37 (2017) 2115-2118. DOI:10.6023/cjoc201701026 |
| [47] |
F. Wang, L. Xu, C. Sun, et al., Appl. Organomet. Chem. 32 (2018) e4505. DOI:10.1002/aoc.4505 |
| [48] |
K.H. Cao, X. Deng, T. Chen, et al., J. Mater. Chem. A 7 (2019) 10918-10923. DOI:10.1039/C9TA00846B |
| [49] |
L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240. DOI:10.1016/j.cclet.2019.04.033 |
| [50] |
S.N. Chu, H.E. Cao, T. Chen, et al., Catal. Commun. 129 (2019) 105730. DOI:10.1016/j.catcom.2019.105730 |
| [51] |
M.X. Liu, Y.M. Li, L. Yu, et al., Sci. China Chem. 61 (2018) 294-299. DOI:10.1007/s11426-017-9158-y |
| [52] |
X.B. Jing, C.Z. Chen, X. Deng, et al., Appl. Organomet. Chem. 32 (2018) e4332. DOI:10.1002/aoc.4332 |
| [53] |
H.E. Cao, M.X. Liu, R.R. Qian, et al., Appl. Organomet. Chem. 33 (2019) e4599. DOI:10.1002/aoc.4599 |
| [54] |
Y.H. Liu, H. Ling, C. Chen, et al., Synlett 30 (2019) 1698-1702. DOI:10.1055/s-0037-1612083 |
| [55] |
H.J. Li, H.E. Cao, T. Chen, et al., 2019, Mol. Catal. 483 (2020) 110715.
|
| [56] |
C.T. Qiu, Y.S. Xu, X. Fan, et al., Adv. Sci. 6 (2019) 1801403. DOI:10.1002/advs.201801403 |
 2020, Vol. 31
2020, Vol. 31 


