b Center for Engineering Training and Basic Experimentation, Heilongjiang University of Science and Technology, Harbin 150022, China
With the increase of energy requirement, most researchers pay more attention to the development of energy storage devices. Compared with lithium-ion batteries [1, 2] and lithium-sulfur batteries [3, 4], supercapacitors [5-7] are widely concerned because of high power density and long cycling life. Furthermore, mechanical flexibility is another important consideration for portable and wearable energy-storage devices. The flexible solid-state supercapacitors have been extensively explored [8, 9], including sandwich-type solid state supercapactiors [10], fibershaped supercapactiors [11] and microsupercapacitors [12-14]. It is worth noting that the electrode material is critical. Therefore, various functional electrode materials, such as carbon materials [15] and metal oxides [16, 17], have been developed. However, it is a challenge to find flexible electrode materials with excellent mechanical properties and high capacitance simultaneously.
In recent years, an emerging family of layered early transition metal carbides and/or nitrides, MXenes, was discovered in 2011 [18]. MXenes can be produced by etching the "A" layers from the MAX phases. Among the MXenes family, Ti3C2Tx is considered as a promising electrode material because of excellent conductivity, high areal capacitance and mechanical flexibility, exhibiting great advantage in the field of energy storage equipment [19-21], especially for supercapacitors [22-25]. However, the irreversible restacking between Ti3C2Tx nanosheets, just like graphene, is inevitable. It is because the strong van der Waals interaction between adjacent Ti3C2Tx nanosheets [24]. The irreversible restacking results in a significant decline of accessible surface area and seriously hinders electrochemical performance. It is well known that the establishment of three-dimensional porous structures has been successfully applied to graphene to alleviate the restacking [26]. By referring to graphene, researchers have prepared Ti3C2Tx aerogels with the help of ethyl-enediamine (EDA), or thiourea dioxide and ammonia solution [21, 27]. However, some of these additives might not be beneficial to high electrochemical performance. For example, the introduction of EDA reduced the cycle stability of the Ti3C2Tx electrode, and partially removed the termination of O, which should be an essential participant in electrochemical reaction in the H2SO4 electrolyte following Eq. S1 (Supporting information) [27, 28]. In this work, we rationally designed and synthesized porous Ti3C2Tx assemblies without any additive by introducing ice as spacers by a facile frozen-drying method. On the one hand, the ice spacers crystallized from the aqueous Ti3C2Tx colloidal suspension could partially prevent the nanosheets from restacking. On the other hand, the ice crystallization in the freeze-drying process might provide an opportunity to tailor the chemistry and properties of Ti3C2Tx and improve their performances in electrochemical applications. This method is also applied to other 2D materials beyond MXene in the field of energy storage and expected to enhance their electrochemical performances [9, 29, 30].
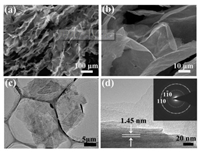
Fig. S1 (Supporting information) demonstrates the fabrication of porous Ti3C2Tx assemblies. The morphology of the porous Ti3C2Tx assemblies was investigated by SEM. As shown in Fig. 1a, it is quite uniform with low magnification, and the Ti3C2Tx assemblies have a three-dimensional porous network structure composed of ultra large Ti3C2Tx lamellar walls and lots of macropores with appropriate porous size of tens of micrometers. Fig. S2 (Supporting information) suggests that there are lots of mesopores in the Ti3C2Tx assemblies. The pore-size dominantly distributes around 4 nm. The specific surface area of the porous Ti3C2Tx assemblies is calculated to be 22.0 m2/g. Fig. 1b presents the faintly wrinkled surface of the Ti3C2Tx lamellar walls, which may induce the generation of mesopores. The lateral size of the lamellar walls of macropores is more than one hundred micrometers. It is much larger than the lateral size of the reported Ti3C2Tx nanosheets (about 3~5 μm) [13, 31]. The ultra large lateral size of the Ti3C2Tx lamellas is helpful for making up a threedimensional porous structure consisting of many macro- and mesopores, which would increase the specific surface area and simultaneously facilitate the infiltration and diffusion of electrolyte ions. The detailed microstructure of the ultra large Ti3C2Tx lamellas separated from the porous assemblies was further investigated by TEM (Fig. 1c). The lamella is constructed by continuously cross-linking small Ti3C2Tx nanosheets, and can provide many active sites, which is important for practical application of supercapacitors. The selected area electron diffraction pattern was tested along the [000 l] zone axis (inset of Fig. 1d).
|
Download:
|
| Fig. 1. (a, b) SEM images, (c, d) TEM images of single Ti3C2Tx lamella separated from the porous assemblies. Inset is the corresponding selected area electron diffraction pattern. | |
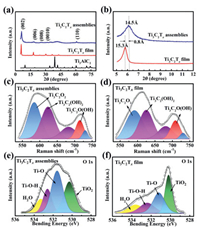
The XRD pattern of the porous Ti3C2Tx assemblies is shown in Fig. 2a. The most intense peak in the XRD pattern of Ti3AlC2 is located at 2θ = 39°, while it disappears in the XRD pattern of the porous Ti3C2Tx assemblies, implying that the Ti3C2Tx has been prepared successfully [27]. According to the characteristic peak of (002) at 6.1°, the interlayer spacing of porous Ti3C2Tx assemblies is calculated to be 14.5 Å (Fig. 2b), which is less than that of Ti3C2Tx film (15.3 Å). It might be caused by the loss of H2O between the Ti3C2Tx layers in the freeze-drying process. The discrepancy in chemical structure between the porous Ti3C2Tx assemblies and the Ti3C2Tx film can be reflected by Raman spectrum. To exclude the influence of the exposure time and the amount of the used sample, the Raman spectra in the range of 530-770 cm–1 for Ti3C2Tx assemblies and Ti3C2Tx film are normalized and shown in Figs. 2c and d, respectively. Peak-differentiating and imitating were carried out using PeakFit software. According to previous report, the peak located at 590 cm–1 is ascribed to the vibrations of the atoms in Ti3C2O2 [28]. In analogy with the case of Ti3C2(OH)2, the intensity of Ti3C2O2 for the porous Ti3C2Tx assemblies is obviously higher than that for Ti3C2Tx film, indicating the more -O functional groups contained in the porous Ti3C2Tx assemblies [28]. The highresolution O 1s spectra of the porous Ti3C2Tx assemblies and Ti3C2Tx film are shown in Figs. 2e and f. The O 1s signal can be deconvoluted into four peaks at 533.5, 532.2, 531.1 and 530.2 eV, corresponding to H2O, Ti-O-H, Ti-O and TiO2, respectively. Their proportion determined from the O 1s spectra is shown in Table S1 (Supporting information). The proportion of the functional group of -O for the porous Ti3C2Tx assemblies is more than that for the Ti3C2Tx film, which reinforces the Raman analysis. The proportion of H2O for the porous Ti3C2Tx assemblies is 10.6%, which is less than that for the Ti3C2Tx film (17.2%). It implies that some H2O molecules intercalated into the interlayers of the porous Ti3C2Tx assemblies might be partially removed.
|
Download:
|
| Fig. 2. (a) XRD patterns, (b) Enlarged XRD patterns, (c, d) Raman spectra of porous Ti3C2Tx assemblies and Ti3C2Tx film. (e, f) O 1s XPS spectra of porous Ti3C2Tx assemblies and Ti3C2Tx film. | |
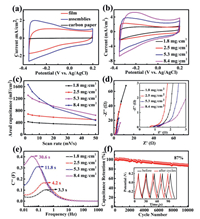
The electrochemical performances of the Ti3C2Tx film and porous Ti3C2Tx assemblies were firstly studied in a three-electrode configuration by using 1 mol/L H2SO4 as electrolyte. Fig. 3a shows the cyclic voltammogram (CV) curves for the porous Ti3C2Tx assemblies and Ti3C2Tx film with same mass loading at the scan rate of 2 mV/s, besides carbon paper. Compared with porous Ti3C2Tx assemblies and Ti3C2Tx film, the CV curve for carbon paper is just a straight line. That means the effective capacitive contribution from carbon paper compared with that from Ti3C2Tx materials can be negligible, and the mass of the carbon paper should not be taken into account as active material when we calculated specific capacitance. The area enclosed by CV curve for the porous Ti3C2Tx assemblies is obviously larger than that for Ti3C2Tx film, which implies that Ti3C2Tx assemblies possess bigger areal and specific capacitance. Fig. S3a (Supporting information) shows the galvanostatic charge-discharge (GCD) curves performed at 1 mA/cm2. The Ti3C2Tx assemblies exhibit higher capacitance retention (72.4%) than Ti3C2Tx film (43.2%) when the scan rate ranges from 2 mV/s to 50 mV/s, as shown in Fig. S3b (Supporting information), which can attribute to the better ability of iondiffusion compared with Ti3C2Tx film (Fig. S3c in Supporting information). After 5000 cycles, the capacitance retention of Ti3C2Tx assemblies is almost the same as that of Ti3C2Tx film (Fig. S3d in Supporting information). On the basis of the above results, the superiority of Ti3C2Tx assemblies than Ti3C2Tx film can be revealed.
|
Download:
|
| Fig. 3. (a) CV curves of carbon paper, Ti3C2Tx film and assemblies. (b) CV curves of Ti3C2Tx assemblies. (c) Variation in areal capacitances of Ti3C2Tx assemblies with scan rates. (d) Nyquist plots and (e) diffusion time plots of Ti3C2Tx assemblies. (f) Cycle stability of Ti3C2Tx assemblies, the inset exhibits the GCD curves of before and after 10000 cycles. | |
In order to explore the impact of mass loadings on capacitance performance, we prepared the Ti3C2Tx assemblies electrodes with different mass loadings (1.8, 2.5, 5.3 and 8.4 mg/cm2). As shown in Fig. 3b, the areal capacitance calculated by integrating the CV curves increases with the loading amount. It is 429.5, 613.3, 1310.0 and 1668.2 mF/cm2, and the corresponding specific capacitance is 238.6, 245.3, 247.2 and 198.6 F/g, respectively. The superiority of the electrode (5.3 mg/cm2) is vividly described by Fig. S4 (Supporting information) [32]. The electrode (8.4 mg/cm2) exhibits the longest discharge time in the GCD curves at 2 mA/cm2 (Fig. S5 in Supporting information). However, its capacitance rapidly declines as the scan rate increasing (Fig. 3c). The capacitance retention for the electrode (8.4 mg/cm2) is calculated to be only 28.5%, and the ones for other three electrodes (1.8, 2.5 and 5.3 mg/ cm2) are 74.1%, 71.9% and 49.3%, respectively. It indicates the good rate performance of the electrodes (1.8 and 2.5 mg/cm2) and the inferior rate capability of the electrodes (5.3 and 8.4 mg/cm2). To illustrate why the capacitance retention declines so fast as mass loading increases, we carried out EIS measurement in a frequency varying from 10 mHz to 200 kHz. The Nyquist curves in Fig. 3d are similar in form with a quasi-semicircle at the high frequency region and a spike at the low frequency region. The inset is the magnified section in the high frequency. The intercept on the Re(Z) is 0.9, 0.7, 0.9 and 1.1 Ω for 1.8, 2.5, 5.3 and 8.4 mg/cm2, respectively. It denotes the equivalent series resistance (Rs). Although they are disparate, they are just about 1.0 Ω. The low Rs indicates the low intrinsic ohmic resistance of the Ti3C2Tx assemblies electrodes. In the high-frequency region, the four charge transfer resistances are almost the same and quite small. The Nyquist plots in the low-frequency region exhibit a slope close to 90° along the imaginary axis for the three electrodes (1.8, 2.5 and 5.3 mg/cm2), while it shows a bigger angle relative to the imaginary axis for the electrode (8.4 mg/cm2). The slope is related to the diffusive resistance of electrolyte in the electrode pores and the proton diffusion in host materials. The smaller slope for the electrode (8.4 mg/cm2) should be due to a higher diffusion resistance. Fig. 3e provides a convenient method to show the influence of frequency on imaginary capacitance. It is a quantitative method to weigh how fast the device can be charged and discharged reversibly. The response time is 3.3, 4.2, 11.8 and 30.6 s for the loading mass of 1.8, 2.5, 5.3 and 8.4 mg/cm2, respectively. It is the reason for the good rate performance of the electrodes (1.8 and 2.5 mg/cm2) and the inferior rate capability of the electrodes (5.3 and 8.4 mg/cm2). Generally, high mass loading causes electrolytic ion to diffuse into the active materials difficulty. An increased loading mass induces more active surface areas of the electrodes to lose efficacy at large current densities, which leads to an increased response time [7]. To evaluate cycling stability, the Ti3C2Tx assemblies electrode was tested through GCD measurements at 20 mA/cm2. Its capacitance is maintained 87% of its initial value after 10000 cycles Fig. 3f). The last 5 cycles show a similar symmetrical triangle shape with the first 5 cycles (insets of Fig. 3f), indicating a remarkable cycling stability of the Ti3C2Tx assemblies electrodes.
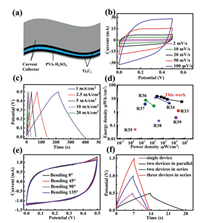
In order to demonstrate the practical application of the porous Ti3C2Tx assemblies, supercapacitors were fabricated by using Ti3C2Tx assemblies for both positive and negative electrodes, and PVA-H2SO4 as electrolyte (Fig. 4a). The CV curves at different scan rates (2~100 mV/s) for the supercapacitor with the total mass loading of 8.3 mg are shown in Fig. 4b. It should be point out that the total mass loadings here is for the two electrodes of the symmetric solid-state supercapacitors, where mass loadings of positive and negative electrode are equal. The rectangular shape of the CV curves can be well maintained even at a high scan rate of 100 mV/s. Fig. 4c displays the GCD curves of the Ti3C2Tx-based supercapacitor at different current density (1-20 mA/cm2). All the curves exhibit perfect linear and symmetrical character, confirming an excellent electrochemical reversibility and a good coulombic efficiency of the Ti3C2Tx-based supercapacitor. The influence of mass loadings on the electrochemical performance of the Ti3C2Txbased supercapacitors is evaluated (Fig. S6 and Table S2 in Supporting information). The power and energy densities of the Ti3C2Tx-based supercapacitor are compared with other supercapacitors reported previously in Fig. 4d [33-39]. When the power density is 250 μW/cm2, our Ti3C2Tx-based supercapacitor exhibits an energy density of 14.7 μWh/cm2; and when the energy density is 5.8 μWh/cm2, the power density is 50 mW/cm2. The Ti3C2Txbased solid-state supercapacitors also exhibit good cycling stability (Fig. S7 in Supporting information). To evaluate the potential use in flexible and wearable electronics, a series of comparative experiments under different bending conditions were conducted on the Ti3C2Tx-based supercapacitors (Fig. S8 in Supporting information). Fig. 4e shows the CV curves under different bending conditions (0°~135°) at 20 mV/s. No obvious changes of the CV curves are observed, indicating an excellent electrochemical stability of the Ti3C2Tx-based supercapacitors. To satisfy the actual demands of energy and power, different working windows and prolonged discharging time could be achieved by the series-parallel connection of the Ti3C2Tx-based supercapacitors. As shown in Fig. 4f, the discharge time of two devices connected in parallel is doubled. Meanwhile, the output voltage of three supercapacitors connected in series is three times as long as that of a single one. In addition, a red light-emitting diode (LED) was lightened by the Ti3C2Tx-based supercapacitors in series after being charged (Fig. S9 in Supporting information).
|
Download:
|
| Fig. 4. Electrochemical properties of the flexible symmetric solid-state supercapacitors. (a) Schematic illustration. (b) CV curves. (c) GCD curves. (d) Ragone plots. (e) CV curves carried out at different bent angles. (f) GCD curves of single device, two devices in parallel, and two or three devices in series. | |
In summary, the three-dimensional porous Ti3C2Tx assemblies were synthesized without any additive by introducing ice as spacers, which deliver a maximum areal capacitance (1668 mF/cm2), an optimized specific capacitance of (247.2 F/g), and excellent capacitance retention (87% after 10000 cycles. In addition, the symmetric solid-state supercapacitors fabricated with the porous Ti3C2Tx assemblies exhibit the maximum power density is 50 mW/cm2. These superior performances might be attributed to the following reasons: (ⅰ) the porous network structure; (ⅱ) the larger specific surface area and more electroactive sites; (ⅲ) the more -O terminations. Therefore, the porous Ti3C2Tx assemblies are promising candidates for electrochemical energy storage devices.
Declaration of competing interestThe authors claim no conflicts of interest.
AcknowledgmentsThis work was partially supported by the National Natural Science Foundation of China (Nos. 11504097, 51772069), the Natural Science Foundation of Heilongjiang Province-China (No. QC2017003), and the Scientific Research Foundation of Heilongjiang Province for Returned Chinese Scholars (Wu Lili).
| [1] |
X.Y. Shan, N. Zhang, R.D. Zheng, H. Gao, X.T. Zhang, Electrochim. Acta 295 (2019) 286-293. DOI:10.1016/j.electacta.2018.10.151 |
| [2] |
Y. Liu, P. Zhang, N. Sun, et al., Adv. Mater. 30 (2018) 1707334. DOI:10.1002/adma.201707334 |
| [3] |
Q. Jin, N. Zhang, C.C. Zhu, H. Gao, X.T. Zhang, Nanoscale 10 (2018) 16935. DOI:10.1039/C8NR05749D |
| [4] |
R.P. Fang, S.Y. Zhao, Z.H. Sun, et al., Adv. Mater. 29 (2017) 1606823. DOI:10.1002/adma.201606823 |
| [5] |
V. Strauss, K. Marsh, M.D. Kowal, M. EI-Kady, R.B. Kaner, Adv. Mater. 30 (2018) 1704449. DOI:10.1002/adma.201704449 |
| [6] |
L.B. Xing, J.L. Zhang, J. Zhang, et al., Electrochim. Acta 176 (2015) 1288-1295. DOI:10.1016/j.electacta.2015.07.150 |
| [7] |
L. Li, N. Zhang, M.Y. Zhang, et al., ACS Sustain. Chem. Eng. 6 (2018) 7442-7450. DOI:10.1021/acssuschemeng.8b00047 |
| [8] |
L.L. Liu, Z.Q. Niu, J. Chen, Chin. Chem. Lett. 29 (2018) 571-581. DOI:10.1016/j.cclet.2018.01.013 |
| [9] |
X.L. Xu, W.H. Shi, W.X. Liu, et al., J. Mater. Chem. A 6 (2018) 24086. DOI:10.1039/C8TA06412A |
| [10] |
Z.Y. Song, D.Z. Zhu, L.C. Li, et al., J. Mater. Chem. A 7 (2019) 1177-1186. DOI:10.1039/C8TA10158B |
| [11] |
L. Chen, D.P. Li, L.N. Chen, et al., Carbon 138 (2018) 264-270. DOI:10.1016/j.carbon.2018.06.022 |
| [12] |
S. Wang, Z.S. Wu, S.H. Zheng, et al., ACS Nano 11 (2017) 4283-4291. DOI:10.1021/acsnano.7b01390 |
| [13] |
P. Li, W.H. Shi, W.X. Liu, et al., Nanotechnology 29 (2018) 445401. DOI:10.1088/1361-6528/aadad4 |
| [14] |
J.Q. Qin, Z.S. Wu, F. Zhou, et al., Chin. Chem. Lett. 29 (2018) 582-58658. DOI:10.1016/j.cclet.2017.08.007 |
| [15] |
J.J. Yoo, K. Balakrishnan, J.S. Huang, Nano Lett. 11 (2011) 1423-1142. DOI:10.1021/nl200225j |
| [16] |
Y. Wang, Y.Z. Zhang, D. Dubbink, et al., Nano Energy 49 (2018) 481-488. DOI:10.1016/j.nanoen.2018.05.002 |
| [17] |
N.R. Chodankar, D.P. Dubal, G.S. Gund, C.D. Lokhande, Electrochim. Acta 165 (2015) 338-347. DOI:10.1016/j.electacta.2015.02.246 |
| [18] |
M. Naguib, M. Kurtoglu, V. Presser, et al., Adv. Mater. 23 (2011) 4248. DOI:10.1002/adma.201102306 |
| [19] |
J.M. Luo, J.H. Zheng, J.W. Nai, et al., Adv. Funct. Mater. (2019) 1808107. |
| [20] |
H. Tang, W.L. Li, L.M. Pan, et al., Adv. Sci. (2018) 1800502. |
| [21] |
X.Y. Wang, Q.S. Fu, J. Wen, et al., Nanoscale 10 (2018) 20828-20835. DOI:10.1039/C8NR06014B |
| [22] |
S.Y. Lin, X.T. Zhang, J. Power Sources 294 (2015) 354-359. DOI:10.1016/j.jpowsour.2015.06.082 |
| [23] |
L. Yu, L. Hu, B. Anasori, et al., ACS Energy Lett. 3 (2018) 1597-1603. DOI:10.1021/acsenergylett.8b00718 |
| [24] |
H.R. Wang, L. Li, C.C. Zhu, et al., J. Alloys Compd. 778 (2019) 858-865. DOI:10.1016/j.jallcom.2018.11.172 |
| [25] |
X.F. Zhang, Y. Liu, S.L. Dong, J.Q. Yang, X.D. Liu, Appl. Surf. Sci. 485 (2019) 1-7. DOI:10.1016/j.apsusc.2019.04.197 |
| [26] |
L.L. Jiang, Z. Fan, Nanoscale 6 (2014) 1922-1945. DOI:10.1039/C3NR04555B |
| [27] |
L. Li, M.Y. Zhang, X.T. Zhang, Z.G. Zhang, J. Power Sources 364 (2017) 234-241. DOI:10.1016/j.jpowsour.2017.08.029 |
| [28] |
M.M. Hu, T. Hu, Z.J. Li, et al., ACS Nano 4 (2018) 3578-3586. |
| [29] |
W.X. Liu, R.L. Yin, X.L. Xu, et al., Adv. Sci. 6 (2019) 1802373. DOI:10.1002/advs.201802373 |
| [30] |
F.F. Wu, X.B. Gao, X.L. Xu, et al., ChemSusChem 13 (2020) 1537-1545. DOI:10.1002/cssc.201903006 |
| [31] |
Q.S. Fu, J. Wen, N. Zhang, et al., RSC Adv. 7 (2017) 11998-12005. DOI:10.1039/C7RA00126F |
| [32] |
W.H. Shi, J. Mao, X.L. Xu, et al., J. Mater. Chem. A 7 (2019) 15654. DOI:10.1039/C9TA04900B |
| [33] |
A. Ramadoss, K.Y. Yoon, M.J. Kwak, et al., J. Power Sources 337 (2017) 159-165. DOI:10.1016/j.jpowsour.2016.10.091 |
| [34] |
H.H. Zhou, H.J. Zhi, G.Y. Han, et al., J.Mater. Sci.Mater. Electron. 27 (2016) 2773-2782. DOI:10.1007/s10854-015-4089-6 |
| [35] |
C.F. Zhang, B. Anasori, A. Seral-Ascaso, et al., Adv. Mater. (2017) 1702678. |
| [36] |
C.Z. Wei, Q. Xu, Z.Q. Chen, et al., Carbohyd Polym. 169 (2017) 50-57. DOI:10.1016/j.carbpol.2017.04.002 |
| [37] |
L. Kou, T.Q. Huang, B.N. Zheng, et al., Nat. Commun. 5 (2014) 3754. DOI:10.1038/ncomms4754 |
| [38] |
Y.L. Shao, J.M. Li, A.G. Li, et al., Mater. Horiz. 4 (2017) 1145-1150. DOI:10.1039/C7MH00441A |
| [39] |
L. Zhang, D.A. Derek, N.T. Alvarez, et al., Small (2017) 1603114. |
 2020, Vol. 31
2020, Vol. 31 

