b Hubei Province Key Laboratory of Coal Conversion and New Carbon Materials, Wuhan University of Science and Technology, Wuhan 430081, China
With the growing problems of energy crisis and environmental pollution, solar energy utilization and conversion is crucial in the sustainable development of human society. Compared to the conventional treatment methods, photocatalytic oxidation technology using eco-friendly semiconductors has been regarded as a promisingway to alleviate theworsening situationin environmental and energy, especially for treating pollutants in water [1-3]. Due to its natural abundance, non-toxicity, excellent chemical stability and photocatalytic activity, TiO2 has been widely used as a semiconductor photocatalyst for organic pollutant degradation. However, the intrinsic wide band gap (~3.2 eV for anatase) leads to its activation only by ultraviolet light (λ < 400 nm), which greatly limits the practical applications of pure TiO2 [4]. Therefore, many strategies have been developed to extend the light absorption range of TiO2 into visible region, such as coupling TiO2 with narrow bad gap semiconductors to form heterostructure. BiOBr is a good candidate for TiO2 to form heterojunctions due to its suitable band gap (~2.7 eV) and chemical stability under visible light irradiation [5-7]. However, the general issue of rapid photo-generated electron-hole recombination still limits the further enhancement of photoactivity for BiOBr.
MXenes, an emerging two-dimensional materials family of early transition metal carbides and carbonitrides, have drawn widespread attention in the field of energy storage, environment remediation and biomedicine. Among the big MXene family, Ti3C2 MXene possesses the most in-depth etching chemistry and detailed theoretical research. Typically, the obtained multilayer Ti3C2 MXene is terminated by -OH, —O and/or —F functional groups (marked as Tx) and the formula is written as Ti3C2Tx [8-10]. Owing to its high electrical conductivity, hydrophilicity and layered structure, Ti3C2Tx is a promising cocatalyst in photocatalysis. Furthermore, Ti3C2Tx can be used as an ideal precursor for the preparation of TiO2/Ti3C2Tx hybrid materials attributed to the uniformly dispersed titanium atoms [11-14]. But the multilayer morphology of Ti3C2Tx affects the absorption of visible light and hinders the light capture by the photocatalyst, resulting in limited utilization of light and low photocatalytic activity [15]. Therefore, it is significant to reduce the thickness of Ti3C2Tx and avoid preventing the light absorption of TiO2.
Herein, 2D titanium carbide (Ti3C2Tx) nanosheets were firstly obtained by chemical exfoliation of commercially available Ti3AlC2 with lithium fluoride and hydrochloric acid. A series of ternary BiOBr/TiO2/Ti3C2Tx nanocomposites were successfully prepared by a facile one-step hydrothermal procedure (presented in the supporting information), which were recorded as BTM-x (x is the theoretical mass ratio of Ti3C2Tx to BiOBr). For comparison, TiO2/Ti3C2Tx hybrids were synthesized via an in-situ oxidation of the delaminated Ti3C2Tx nanosheets and pure BiOBr was also obtained via the similar hydrothermal process. Detailed synthesis procedure, characterization and photocatalytic evaluation were given in the supplementary information. The morphology, crystal structure, optical property and visible-light photocatalytic activity of the as-synthesized composites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), highresolution transmission electron microscopy (HRTEM), UV–vis diffuse reflectance spectra (UV–vis DRS), photoluminescence spectra (PL), respectively. Moreover, a possible photocatalytic mechanism of BiOBr/TiO2/Ti3C2Tx composites was proposed.
The X-ray diffraction patterns of pristine Ti3AlC2, Ti3C2Tx, TiO2/Ti3C2Tx, BiOBr and BiOBr/TiO2/Ti3C2Tx composites were recorded in the 2θ range of 5°~90° as illustrated in Fig. 1. After etching with lithium fluoride and hydrochloric acid, the characteristic diffraction peaks of Ti3AlC2 disappear. Meanwhile, the characteristic peaks according to (002) crystal plane is broadened and shifted from 9.8° to 6.2°, suggesting the successful exfoliation of Al atoms from the interlayers and the formation of Ti3C2Tx MXene [16]. After in situ oxidation via hydrothermal process, several new diffraction peaks at 2θ = 25.4°, 37.9°, 48.2°, 54.1°, 55.2°, 62.9° emerge in TiO2/ Ti3C2Tx, which are in good accordance with the (101), (004), (200), (105), (211) and (204) planes of the anatase phase of TiO2 (JCPDS No. 73-1764), respectively. Besides, the diffraction peak ascribed to the (002) crystal plane of Ti3C2Tx is still observable but presents relatively lower intensity than pristine Ti3C2Tx, which demonstrates the residue of Ti3C2Tx MXene and part transformation to TiO2 [17, 18]. As shown in Fig. 1, typical peaks of the pure BiOBr were indexed as the tetragonal BiOBr (JCPDS No. 09-0393) without any crystalline impurity. For the BiOBr/TiO2/Ti3C2Tx composites, it can be observed that the co-exist of the diffraction peaks of BiOBr and TiO2 in all the composites [19-21]. However, it is hard to observe any peaks of Ti3C2Tx, which may be explained by the relatively low content of Ti3C2Tx.

|
Download:
|
| Fig. 1. XRD patterns of Ti3AlC2, Ti3C2Tx, TiO2/Ti3C2Tx, BiOBr and BiOBr/TiO2/Ti3C2Tx composites. | |
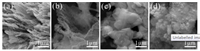
The morphology of the Ti3C2Tx, TiO2/Ti3C2Tx, BiOBr and BTM-20% composites were characterized by SEM and HRTEM. Fig. 2a reveals the typical lamellar morphology of Ti3C2Tx MXene, confirming the Ti3AlC2 was successfully exfoliated to form Ti3C2Tx nanosheets. After the hydrothermal treatment, plenty of TiO2 nanoparticles anchor on the Ti3C2Tx sheets (Fig. 2b). As demonstrated in Figs. 2c and d, the smooth nanoplates of BiOBr are evolved into rough and covered with numerous TiO2 nanoparticles on the surface of the BiOBr. The lamellar structureof BiOBr is retained during its coupling with TiO2/Ti3C2Tx, which is consistent with the XRD results. HRTEM images of BTM-20% composites in Figs. 3a and b further show the distribution of TiO2 and BiOBr in the hybrid composite2. Combining with the HRTEM images and EDS elemental mappings of BiOBr/TiO2/Ti3C2Tx composites (Fig. S1 in Supporting information), it can be confirmed that the TiO2 particles accompanied with residual Ti3C2Tx lamella are distributed over the BiOBr lamella uniformly. As exhibition in Figs. 3c and d, the corresponding lattice fringe value of 0.35 nm and 0.28 nm coincides well with the (101) anatase crystal plane of TiO2 and the (110) plane of BiOBr, respectively [22]. Combining with XRD results, it proves that there have realized ideal interfacial contact and construction of heterojunction between TiO2 and BiOBr.
|
Download:
|
| Fig. 2. SEM images of (a) Ti3C2Tx (b) TiO2/Ti3C2Tx (c) BiOBr and (d) BTM-20%. | |

|
Download:
|
| Fig. 3. (a, b) TEM images of BTM-20 %; (c, d) HRTEM of BTM-20%. | |
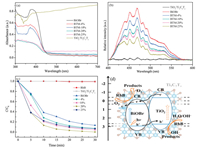
The UV–vis diffuse reflectance spectra (UV–vis DRS) and photoluminescence (PL) spectra were performed to analyze the optical properties of the as-synthesized nanocomposite. As shown in Fig. 4a, pure BiOBr exhibits a strong light response in ultraviolet region and its absorption edge for visible light is around 460 nm which corresponds to a band gap of 2.7 eV [23]. Meanwhile, TiO2/Ti3C2Tx shows higher visible absorption due to the essentially dark Ti3C2Tx. Compared with BiOBr, the UV–vis absorption edges of BiOBr/TiO2/Ti3C2Tx composites occur an obvious red shift and show enhanced absorption for visible light due to the synergistic effect between BiOBr and TiO2. This means BiOBr/TiO2/Ti3C2Tx composites would be more effectively utilize solar energy than pure BiOBr, which is beneficial for improving photocatalytic efficiency.
|
Download:
|
| Fig. 4. (a) UV–vis DRS and (b) PL spectra of BiOBr, TiO2/Ti3C2Tx and BiOBr/TiO2/Ti3C2Tx composites. (c) Visible-light photocatalytic degradation of RhB solution over BiOBr, TiO2/Ti3C2Tx and BiOBr/TiO2/Ti3C2Tx composites. (d) The proposed charge separation and transfer pathways in the BiOBr/TiO2/Ti3C2Tx system under visible-light irradiation. | |
To analyze the recombination details of photo-generated electrons and holes, room temperature PL spectra were carried out with an excitation wavelength of 325 nm. Fig. 4b shows the PL spectra of BiOBr, TiO2/Ti3C2Tx and BiOBr/TiO2/Ti3C2Tx composites. There is no doubt that TiO2/Ti3C2Tx and BiOBr/TiO2/Ti3C2Tx composites display much lower PL emission intensities than BiOBr, indicating their recombination of photo-generated electron-hole pairs is effectively inhibited [24]. It is rationally attributed by the remarkable conductivity of Ti3C2Tx and band structure matching between BiOBr and TiO2.
As shown in Fig. 4c, the photocatalytic activities of the asprepared samples were evaluated by the degradation of rhodamine B solution(RhB, 100 mg/L)under visible light irradiation(λ≥420 nm). For eliminating the effect of absorption, all samples experienced a control test to get adsorption/desorption equilibrium in a dark condition before irradiation with visible light. It is obvious that TiO2/Ti3C2Tx hybrids show barely photocatalytic activity for RhB under visible light irradiationwithin 30 min. Moreover, BiOBr/TiO2/Ti3C2Tx nanocomposites present higher photocatalytic activity than pure BiOBr and TiO2/Ti3C2Tx, especially BTM-20% possesses 99.8% degradation rate for RhB within 30 min. Besides, the x value has a conspicuous effect on the photocatalytic activity and there is an optimal value. This is mainly due to the synergistic effects in the BiOBr/TiO2/Ti3C2Tx nanocomposites and ternary components play different roles in photocatalytic process. The recycling stability of BTM-20% during degradation of rhodamine B is presented in Fig. S3 (Supporting information). After five consecutive cycles, BTM-20% could keep 95.6% degradation rate, which indicates its good recyclability of the photocatalyst.
Based on the above results and discussion, a possible mechanism to explain the outstanding photocatalytic activity of BiOBr/TiO2/Ti3C2Tx nanocomposites is elucidated. On the one hand, the absorption edges of BiOBr/TiO2/Ti3C2Tx nanocomposites further extend to visible-light region and their absorption intensities enhance compared with BiOBr, which facilitates to harvest solar energy. On the other hand, the ternary heterojunction structure may generate a remarkable synergistic effect, leading to the efficient separation and transfer of photo-generated electrons and holes. As illustrated in Fig. 4d, the band gap of TiO2 (~3.2 eV) is wider than that of BiOBr (~2.7 eV) and the corresponding conductive band (CB) and valence band (VB) positions of TiO2 are both more negative than those of BiOBr. The intimate contact between BiOBr and TiO2 may construct a heterojunction. As mentioned, TiO2 shows only little visible light response due to its larger band gap, BiOBr can be excited easily by visible light and produce photo-induced electron-hole pairs. The construction of heterojunction between BiOBr and TiO2 makes it rational that the photo-generated holes will rapidly migrate from the VB of BiOBr to VB of TiO2 and the excited electrons will also transfer from the CB of TiO2 to BiOBr under visible-light irradiation, leading to an efficient separation and transfer of photo-generated electrons and holes [25]. What is more, the remaining Ti3C2Tx in the ternary BiOBr/TiO2/Ti3C2Tx hybrids can serve as an efficient electron trap due to its intrinsic conductivity to further inhibit the photoinduced charge recombination [26]. The photo-generated electrons on the CB of BiOBr and TiO2 will fast transfer to Ti3C2Tx. The holes accumulated in the valence band of TiO2 possess outstanding ability to directly oxidize the pollutants adsorbed on the photocatalyst surface. Meanwhile, abundant holes can also react with OH- groups and H2O molecules to produce •OH radicals. Superoxide radical anions (•O2-) may also emerge from the reaction of the electrons in the CB of BiOBr with oxygen molecules absorbed on the surface of materials or dissolved in water [27-30]. Subsequently, both •OH and •O2- could act as active species to effectively degrade RhB.
In this work, a series of ternary BiOBr/TiO2/Ti3C2Tx composites with heterojunction structure have been designed and constructed by a facile one-step hydrothermal process. The as-prepared ternary composites show higher visible-light photocatalytic activities for the degradation of RhB than pure BiOBr and TiO2/ Ti3C2Tx due to the optimized synergetic effects of BiOBr, TiO2 and Ti3C2Tx. It is found that there is a contact interface of heterojunction between BiOBr nanoplates and TiO2 nanoparticles. The unique heterojunction structure not only enhance the absorption for visible light, but also promote the transfer of photo-excited electrons and holes due to the well-matching of energy band position between BiOBr and TiO2. The introduction of Ti3C2Tx serves as an efficient electron trap and accelerates the migration and transfer of photo-excited electrons. This work may provide reference of utilizing MXene as novel co-catalytic material to achieve highly efficient, steady and cost-effective semiconductor photocatalysts.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51472186, 51902232, 51402221) and the China Scholarship Council Fund (No. 201708420210).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.038.
| [1] |
Z. Xing, J. Zhang, J. Cui, et al., Appl. Catal. B:Environ. 225 (2018) 452-467. DOI:10.1016/j.apcatb.2017.12.005 |
| [2] |
T. Yang, J. Peng, Y. Zheng, et al., Appl. Catal. B:Environ. 221 (2018) 223-234. DOI:10.1016/j.apcatb.2017.09.025 |
| [3] |
L. Lu, T. Jiang, W.S. Jing, G.W. Zhou, H.X. Shi, Chem. Lett. 47 (2018) 613-616. DOI:10.1246/cl.180080 |
| [4] |
K. Qi, B. Cheng, J. Yu, W. Ho, Chin. J. Catal. 38 (2017) 1936-1955. DOI:10.1016/S1872-2067(17)62962-0 |
| [5] |
C. Xue, T. Zhang, S. Ding, J. Wei, G. Yang, ACS Appl. Mater. Interfaces 9 (2017) 16091-16102. DOI:10.1021/acsami.7b00433 |
| [6] |
Z.D. Wei, R. Wang, Chin. Chem. Lett. 27 (2016) 769-772. DOI:10.1016/j.cclet.2016.03.013 |
| [7] |
M. Ji, Z. Zhang, J. Xia, et al., Chin. Chem. Lett. 29 (2018) 805-810. DOI:10.1016/j.cclet.2018.05.002 |
| [8] |
Z. Li, Y. Wu, Small (2019) 1804736. |
| [9] |
G. Gao, A.P. O'Mullane, A. Du, ACS Catal. 7 (2016) 494-500. |
| [10] |
Y. Yang, S. Umrao, S. Lai, S. Lee, J. Phys. Chem. Lett. 8 (2017) 859-865. DOI:10.1021/acs.jpclett.6b03064 |
| [11] |
K. Yan, Y. Guan, Y. Cong, et al., Chin. J. Inorg.Chem. 35 (2019) 1203-1211. |
| [12] |
B. Ahmed, D.H. Anjum, M.N. Hedhili, Y. Gogotsi, H.N. Alshareef, Nanoscale 8 (2016) 7580-7587. DOI:10.1039/C6NR00002A |
| [13] |
C. Peng, X. Yang, Y. Li, et al., ACS Appl. Mater. Interfaces 8 (2016) 6051-6060. DOI:10.1021/acsami.5b11973 |
| [14] |
X. Zhang, Y. Liu, S. Dong, Z. Ye, Y. Guo, Ceram. Int. 43 (2017) 11065-11070. DOI:10.1016/j.ceramint.2017.05.151 |
| [15] |
W. Yuan, L. Cheng, Y. Zhang, et al., Adv. Mater. Interfaces 4 (2017) 1700577. DOI:10.1002/admi.201700577 |
| [16] |
J. Zhu, Y. Tang, C. Yang, F. Wang, M. Cao, J.Electrochem.Soc. 163 (2016) A785-A791. DOI:10.1149/2.0981605jes |
| [17] |
Y. Gao, L. Wang, A. Zhou, et al., Mater. Lett. 150 (2015) 62-64. DOI:10.1016/j.matlet.2015.02.135 |
| [18] |
C. Peng, H. Wang, H. Yu, F. Peng, Mater. Res. Bull. 89 (2017) 16-25. DOI:10.1016/j.materresbull.2016.12.049 |
| [19] |
Q.X. Xia, N.M. Shinde, J.M. Yun, et al., Electrochim. Acta 271 (2018) 351-360. DOI:10.1016/j.electacta.2018.03.168 |
| [20] |
X. Yu, J. Shi, L. Feng, C. Li, L. Wang, Appl. Surf. Sci. 396 (2017) 1775-1782. DOI:10.1016/j.apsusc.2016.11.219 |
| [21] |
T. Jiang, J. Li, Z. Sun, et al., Ceram. Int. 42 (2016) 16463-16468. DOI:10.1016/j.ceramint.2016.06.079 |
| [22] |
J. Rashid, A. Abbas, L.C. Chang, et al., Sci. Total Environ. 665 (2019) 668-677. DOI:10.1016/j.scitotenv.2019.02.145 |
| [23] |
A.M. Alansi, M. Al-Qunaibit, I.O. Alade, T.F. Qahtan, T.A. Saleh, J. Mol. Liq. 253 (2018) 297-304. DOI:10.1016/j.molliq.2018.01.034 |
| [24] |
X. Li, C. Dong, K.L. Wu, et al., Mater. Lett. 164 (2016) 502-504. DOI:10.1016/j.matlet.2015.10.128 |
| [25] |
X. Tan, X. Li, T. Yu, Y. Zhao, Trans. Tianjin Univ. 22 (2016) 211-217. DOI:10.1007/s12209-016-2778-8 |
| [26] |
C. Liu, Q. Xu, Q. Zhang, et al., J. Mater. Sci. 54 (2019) 2458-2471. DOI:10.1007/s10853-018-2990-0 |
| [27] |
C. Xue, X. Xu, G. Yang, S. Ding, RSC Adv. 5 (2015) 102228-102237. DOI:10.1039/C5RA20510G |
| [28] |
W. Wang, Z. Zeng, G. Zeng, et al., Chem. Eng. J. 378 (2019) 122132. DOI:10.1016/j.cej.2019.122132 |
| [29] |
Y. Yang, Z. Zeng, G. Zeng, et al., Appl. Catal. B:Environ. 258 (2019) 117956. DOI:10.1016/j.apcatb.2019.117956 |
| [30] |
H. Yi, M. Jiang, D. Huang, et al., J. Taiwan Inst. Chem. E 93 (2018) 184-192. DOI:10.1016/j.jtice.2018.06.037 |
 2020, Vol. 31
2020, Vol. 31 

