b School of Engineering Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
As a promising next-generation electrochemical energy storage device, lithium-ion capacitors (LICs) combine a capacitive cathode (ions sorption) and a faradaic anode (Li+ insertion) in a hybrid configuration using nonaqueous Li-containing electrolyte [1-5]. The introduction of battery-type anode not only enhances the theoretical specific capacitance but also elevates the operating voltage, thus providing an energy density 3–4 times higher than conventional symmetric supercapacitors [6-9]. After the first prototype proposed by Amatucci et al. [10], various LIC systems using different anodes have been reported with encouraging performances, including Li4Ti5O12 [11], H2Ti6O13 [12], V2O5 [13], TiO2 [14], Nb2O5 [15], etc. However, these traditional battery-type anodes usually suffer from poor electron transportation and sluggish ion intercalation during the repeated charge and discharge, which will neutralize their large capacity and impair the long-term electrochemical stability [16]. In this regard, materials with high electrical conductivity and large interlayer spacing would be promising electrode materials.
Recently, a new family of layered transition metal carbides and nitrides, commonly referred to as MXenes, have shown great potential in energy-related fields due to their high electrical conductivity, layered structure, large interlayer spacing and rich surface/terminal functional groups [17-21]. MXenes are usually synthesized by selectively etching of layered MAX ceramics, in which M is an early transition metal, A is a III or IV A-group element and X is C or N. As a result, MXenes have a general formula of Mn+1XnTx (n = 1–3), where Tx stands for the different surface terminations (hydroxyl, oxygen, or fluorine) [22-24]. Up till now, MXenes such as Ti3C2Tx, Nb2CTx [25], Nb4C3Tx [26] and Mo2CTx [27], have been investigated as pseudocapacitive anode in nonaqueous half-cell, demonstrating a stable potential range of 0–3 V vs. Li/Li+ and high gravimetric capacity of 100–800 mAh/g. Consequently, MXene anodes coupled with carbon cathodes are also anticipated to further enhance the overall energy storage capability of LICs. For example, Gogotsi et al. [28] assembled full-cell LICs using Nb2CTx MXene and carbon nanotubeas compositeanode, which can deliver a maximumgravimetric energy densitiesof 49 Wh/kg.Tao et al. [29] adopted a pillared structure design of Ti3C2Tx MXene with enlarged interlayerspacing toachieve a highenergy densityof 105.56 Wh/kg. Bipolar Ti3C2Tx MXene is also reported to enable the construction of an aqueous LIC with enhanced voltage window of operation [30]. However, the energy performances at higher rates in these reports are still not satisfactory mainly because of the mismatched kinetics between sluggish lithium intercalation in anode and rapid adsorption of ions at the cathode interface[31, 32]. For this reason, it is urgent to exploit appropriate MXene materials with improved kinetics to achieve both efficient energy storage and excellent rate performance.
Synthesis of electrode materials with open structures and highly crystallinity has been proven to be an effective way to boost the electrochemical performance, which could efficiently improve the electrolyte diffusion and electron transfer [33-38]. However, the control of MXene nanostructures still remains a great challenge because the exfoliated MXene layers can easily restack into bulk state and severely impede electrolyte diffusion [39, 40]. Moreover, the crystallinity of MXene layers is usually hampered by the fractured morphology induced during the delamination processes, causing poor electron transportation and high contact resistance [19]. Thus, the crucial issue for the application of MXenes in LICs is to simultaneously regulate the morphologies and crystal structures.
In this work, we present a simple method involving combustion synthesis and acid treatment to prepare accordion-like Ti3C2Tx MXene with open structure and high crystallinity and explore its energy storage capabilities as anode materials in LICs. Due to the improved ion diffusion and electron transportation of Ti3C2Tx MXene anode, the unbalanced electrode kinetics in LICs can be largely overcome to acquire an enhanced power performance. The assembled Ti3C2Tx-based LICs provides a maximum energy density of 106 Wh/kg and still exhibits a superior energy density of 79 Wh/kg even at a higher power density of 5.2 kW/kg.
A facile combustion synthesis in Ar atmosphere was adopted to prepare Ti3AlC2 precursor. Typically, Ti powders, Al powders and C were well-mixed by ball-milling with a mass ratio of 3:1.4:1.9. Then the powders were placed in graphite crucible and initiated the combustion synthesis in a sealed reactor. The whole reaction took only a few of seconds, and the theoretical reaction temperature is estimated to be 3450 K according to the method used in our previous study [9]. After cooling to room temperature, the produced Ti3AlC2 precursor was subsequently ball-milled at a speed of 300 r/min for 5 h to reduce the particle size. Then 2 g Ti3AlC2 powder were immersed in 30 mL 40% HF solution and stirred at 40 ℃ for 24 h under Ar protection. After HF etching, the suspension was centrifuged at 9000 rpm to separate the powders from the supernatants and washed with sufficient deionized water. Finally, the delaminated Ti3C2Tx MXene was freeze-dried to obtain powders.
X-ray diffraction (XRD) patterns were done on a Bruker D8 Xray diffractometer using monochromatic Cu Kα radiation (λ =1.54060 Å). Zeiss SIGMA scanning electron microscope was adopted to conduct Scanning electron microscopy (SEM). Transmission electron microscopy (TEM) characterization was conducted on a JEOL JEM-2010 equipped with selected area electron diffraction (SAED).
The fabrication of Ti3C2Tx MXene anode was carried out by a blade-casting method. To prepare electrode slurries, the active materials were well-mixed with carbon black (Alfa Aesar) and poly vinylidene fluoride (PVDF, Alfa Aesar) in weight ratios of 8:1:1 in Nmethyl-2-pyrrolidone (NMP, Sigma) solvent. The slurries were uniformly coated onto Cu foils (thickness: 15 mm) and dried in a vacuum oven overnight at 120 ℃ for 24 h. For cathode preparation, activated carbon (YP50, Kuraray chemicals, Japan), carbon black (Alfa Aesar) and polyvinylidene fluoride (PVDF, Alfa Aesar) in weight ratios of 8:1:1 were thoroughly mixed in NMP to form a slurry, which was then coated onto Al foils (thickness: 25 μm) and dried at 120 ℃ in vacuum for 24 h. The typical mass loading for anode and cathode in this work is 1.5 and 4.5 mg/cm2, respectively.
CR2032-type coin cells were assembled in an argon-filled glove box (MBraun, Inc.). Celgard 2400 was used as the separator, and 1 mol/L LiPF6 in ethylene carbonate, diethyl carbonate and dimethyl carbonate (EC/DEC/DMC, v/v/v = 1:1:1) was used as electrolyte. Before LIC full-cell fabrication, the MXene anode was prelithiated at 0.1 A/g within 0.03–3 V for 10 cycles to eliminate the irreversible side reactions. Cyclic voltammetry (CV), galvanostatic chargedischarge (GCD) and electrochemical impedance spectroscopy (EIS) tests were conducted using a BioLogic VMP3 electrochemical analyzer under ambient conditions. EIS was performed from 100 mHz to 100 kHz with an amplitude of 10 mV. Arbin MSTAT4 electrochemical station was employed to carry out cyclic stability test.
The specific capacitance C (F/g, normalized on the total mass of electrodes) was calculated from the discharge curve using:
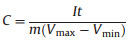
|
where I (mA) stands for the current in GCD test, t (s) is the discharge time, m (mg) is the total mass of electrode, Vmax and Vmin (V) are the upper and lower voltage limit of discharge procedure. The energy density E (Wh/kg) and power density P (kW/kg) were calculated by the following formulas:
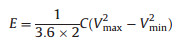
|
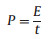
|
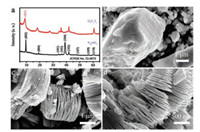
The XRD patterns of Ti3AlC2 precursor and delaminated Ti3C2Tx MXene are presented in Fig. 1a. The successful fabrication of pure Ti3AlC2 phase by combustion synthesis can be easily validated, which is in accordance with the standard spectrum (JCPDS No. 52- 0875). After HF etching, the strong (104) peak at 39 ℃ in the out-of-plane direction of Ti3AlC2 was significantly weakened, suggesting the removal of Al layers in the bulk [41]. Moreover, the broadened peak of (002) appeared at 8.7° in Ti3C2Tx indicates the substantial expansion of the interlayer spacing from 9.3 Å for Ti3AlC2 to 10.1 Å for Ti3C2Tx according to Bragg equation (2d sinθ = nλ), which would reduce the resistance of ion diffusion and leave enough room for rapid Li ion intercalation.
|
Download:
|
| Fig. 1. (a) XRD patterns of Ti3AlC2 precursor and Ti3C2Tx MXene by HF etching; (b) SEM image of pristine Ti3AlC2; (c, d) SEM pictures of layered Ti3C2Tx MXene. | |
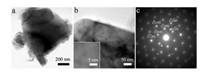
SEM images further revealed the good delamination of MXene from it bulk state precursor. A typical SEM image of Ti3AlC2 in Fig. 1b clearly gives a particle morphology with dimension around 8–10 μm, and it is interesting to find a seamless stacked feature in Ti3AlC2 precursor, suggesting the appropriate removal and delamination of Al layers could result in the successful exfoliation of MXene products. As expected, the Ti3C2Tx MXene (Fig. 1c) after selective etching presents a typical accordion-like morphology with many nano- and submicron- scale spaces between the flakes, provide important channels for swift ion diffusion into the bulk of electrode especially at high rates. A magnified SEM image of Ti3C2Tx (Fig. 1d) shows the distance between adjacent Ti3C2Tx nanoflakes is 20–30 nm and the average thickness of MXene layers is around 10 nm. TEM picture of Ti3C2Tx in Fig. 2a displays the high quality of exfoliated MXene nanoflakes with distinct edges and lateral sizes of several micrometers. The high-resolution TEM (HRTEM) images (Fig. 2b and inset) confirms the good crystallinity of Ti3C2Tx with a measured (002) plane spacing of 1.01 nm, which is consistent with the XRD results. The high crystallinity of Ti3C2Tx can also be validated from the highly symmetric hexagonal pattern in SAED results (Fig. 2c). The above structural and morphological analysis confirms open structure and high crystallinity can be maintained in Ti3C2Tx MXenes prepared in this work, which may benefit ion diffusion, charge transfer and electron transportation under quick charge/discharge cycles. Therefore, we further explored its electrochemical performance as LIC anode.
|
Download:
|
| Fig. 2. (a, b) TEM images of Ti3C2Tx MXene nanolayers (inset in b is a magnified TEM picture indicating the interlayer spacing); (c) SAED of delaminated Ti3C2Tx MXene. | |
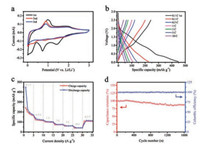
The electrochemical behavior of Ti3C2Tx MXene as LIC anode was investigated using coin-type half-cells between 0.03 V and 3 V vs. Li/Li+. Fig. 3a shows the initial three CV curves of Ti3C2Tx at a scan rate of 0.1 mV/s. It can be clearly observed a sharp reduction peak appears at about 0.8 V in the first lithiation cycle, which is ascribed to the deposition of solid electrolyte interface (SEI) layer on electrode surface [42]. The small reduction peak at around 1.5 V is probably caused by the side reactions between the functional groups in Ti3C2Tx MXene and the electrolyte [43]. The oxidation peaks at around 0.8–1 V in the first three CV cycles is probably caused by the reversible desertion of Li+ from the bulk of MXene anode and several oxidative side reactions. However, this oxidation peak gradually fades as the CV test continues, suggesting an electrochemical equilibrium is being reached within the initial activation process. In the following two cycles, Ti3C2Tx anode exhibits quasi-rectangular shaped CV curves corresponding to typical capacitive behavior, meaning the completion of stable SEI formation. In Fig. 3b, an initial discharge capacity of 450 mAh/g and a charge capacity of 250 mAh/g can be obtained for Ti3C2Tx anode at the current density of 0.1 C (1 C equals 260 mA/g). The low initial Coulomb efficiency is generally attributed to the irreversible consumption of electrolyte during the formation of SEI films. However, all other GCD curves of Ti3C2Tx at different current densities display linear feature and show no obvious plateaus, indicating a stable Li-storing behavior after the first lithiation. This phenomenon should be attributed to the open structure and high crystallinity of Ti3C2Tx nanoflakes allowing fast diffusion of the Li+ ions and efficient charge transfer at the electrode/electrolyte interface.
|
Download:
|
| Fig. 3. (a) The first three CV curves of Ti3C2Tx MXene anode at the scan rate of 0.1 mV/s within 0.01–3 V vs. Li/Li+; (b) GCD results of Ti3C2Tx MXene at different current densities; (c) Rate performance of Ti3C2Tx MXene; (d) Long-term electrochemical stability of Ti3C2Tx MXene at 5 C for 1600 cycles. | |
The rate capability of Ti3C2Tx anode is given in Fig. 3c. Ti3C2Tx anode can achieve a high capacity of 236, 135, 103, 80 and 65 mAh/g at 0.1, 0.5, 1, 3 and 5 C, respectively, providing superior performances when compared with other reported MXene materials, including V4C3 nanolayers (225 mAh/g) [44], exfoliated Ti2C (110 mAh/g) [18] and multilayered Ti3C2Tx (123 mAh/g) [45]. After 1600 cycles at 5 C, the Ti3C2Tx electrode still delivers a reversible capacity of 119 mAh/g (Fig. 3d), corresponding to a good capacity retention of 88%. More importantly, the Coulombic efficiency during the whole cycling test is higher than 99%.
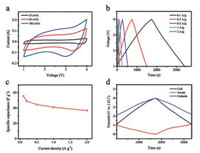
Ti3C2Tx anode was assembled with commercial AC cathode to construct full-cell using nonaqueous 1 mol/L LiPF6 electrolyte. Before fabricating LIC device, pristine Ti3C2Tx anode was first prelithiated for 5 cycles at 0.1 A/g from 0.03 V to 3 V to eliminate the irreversible capacity and stabilize anode potential. To balance the mismatched capacity at both electrodes, the mass ratio of cathode and anode in the full-cell was optimized as 3:1. In Fig. 4a, the CV curves of AC//Ti3C2Tx LIC at various scan rates present typical rectangular shape within the voltage range of 1–4 V, indicating a capacitance-dominated energy storage behavior. The slight deviation at higher voltage is caused by the different energy harvesting mechanism between capacitive cathode and intercalative anode [46]. In the charging process, Li+ ions are inserted into Ti3C2Tx anode and the potential of this battery-type electrode declines. On the cathode part, ions are absorbed at the porous surface to elevate the voltage of this capacitor-type electrode [47]. The movement of ions is in the reverse direction during the discharge process. The GCD profiles at various current densities are given in Fig. 4b. The highly symmetric linear charge and discharge curves can be clearly observed, also confirming the capacitive feature of AC//Ti3C2Tx. A good rate performance can be observed in Fig. 4c, since the capacitance of LIC (based on the total mass of both electrodes) at 0.1 A/g is 56 F/g and still keeps 69% of this value even as the current density increases twenty times to 2 A/g. The potential change of cathode and anode during the charge/ discharge process are also explored using a Li foil as reference electrode and the results are shown in Fig. 4d. The low potential of Ti3C2Tx anode in the range of 0.03–1.03 V is helpful to fully utilize its high capacity, while the charge/discharge profiles of cathode can exhibit triangular shape with high symmetry.
|
Download:
|
| Fig. 4. (a) CV curves, (b) GCD lines and (c) rate capability of AC//Ti3C2Tx LIC; (d) Change of cathode and anode potential of AC//Ti3C2Tx during charge and discharge. | |
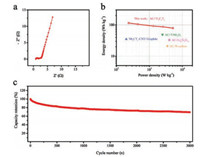
EIS technique was used to give insights of ion transport properties within AC//Ti3C2Tx. The Nyquist plot in Fig. 5a features a miniature semicircle in high-frequency part, suggesting the greatly reduced charge-transfer resistance (Rct). The sloping line in the middle frequency range indicates the Warburg impendence (Zw) in the electrode channels [48]. The internal resistance (Rs) obtained from the intercept of Nyquist plot at the real axis is 0.86 Ω, which is the combination of ionic resistance, electronic resistance of current collector and contact resistance between electrode material and current collector [49, 50]. The low equivalent series resistance (ESR) of 2.02 Ω by extrapolating the semicircle to the real axis also manifests the mitigated ion diffusion resistance across the electrolyte/electrode interface [51]. The Ragone diagram of AC// Ti3C2Tx LIC is shown in Fig. 5b. A high energy density of 106 Wh/kg can be achieved at the power density of 0.23 kW/kg (based on the total mass of active materials in electrode), which is superior than recent reports on MXene-based LIC systems, including AC//Ti2C (30 Wh/kg) [52] and Nb2CTx-CNT//graphite (49 Wh/kg) [28]. This greatly improved energy-storage performance further indicates the employment of accordion-like Ti3C2Tx MXene proposed in this work is helpful to relieve the mismatched kinetics between cathode and anode in conventional LICs. Even with a high power density of 5.2 kW/kg, AC//Ti3C2Tx can still deliver provide a sizeable energy density of 79 Wh/kg. The capability of AC//Ti3C2Tx to keep high energy at high power also surpasses other LIC systems, such as AC//N-doped carbon sphere (21 Wh/kg@3.5 kW/kg) [53], AC// TiNb2O7 nanotube (35 Wh/kg@7 kW/kg) [54] and biomass-derived AC//Li4Ti5O12 (33 Wh/kg@4 kW/kg) [11]. In addition, AC//Ti3C2Tx presents a reasonable capacity retention of 71% after 3000 cycles at 2 A/g and the Columbic efficiency keeps nearly 100% throughout the cycling test (Fig. 5c), further indicating its efficient energy utilization during long-term operation at quick rates. Moreover, the capacity fading issue can be effectively alleviated by incorporating highly conducting networks such as carbon nanotube, graphene and carbon nanofibers [28, 55-57].
|
Download:
|
| Fig. 5. (a) Nyquist plot of AC//Ti3C2Tx LIC; (b) Ragone plot of AC//Ti3C2Tx and several typical MXene-based LICs; (c) Cyclic performance of AC//Ti3C2Tx for 3000 cycles. | |
Our study provides a new platform to employ accordion-like Ti3C2Tx MXene materials with porous and crystalline feature to acquire both high energy and power densities, which can be ascribed to the following aspects: (i) The porous space between the layers in accordion-like Ti3C2Tx anode offers multi-channel pathway for swift ion diffusion during lithium insertion/extraction reaction, thus ensuring efficient energy harvesting especially at high rates; (ii) The high crystallinity of Ti3C2Tx provides facile electron transport highway to boost charge transfer and reduce interface resistance; (iii) The unbalanced electrode kinetics in LIC full-cell is largely improved through regulating the potential range of AC cathode and Ti3C2Tx anode.
In summary, accordion-like Ti3C2Tx MXene with sufficient porosity and high conductivity has been fabricated by a facile liquid-phase exfoliation method using a MAX precursor obtained from combustion synthesis. Due to its distinguishing morphological and structural features, Ti3C2Tx anode exhibits a high capacity of 236 mAh/g and capacity retention of 88% after 1600 cycles, which is a significant enhancement towards Li+ ion storage in nonaqueous electrolytes. Assembled with commercial AC cathode, the Ti3C2Tx-based LIC model system is capable of delivering a superior maximum energy density of 106 Wh/kg and power density of 5.2 kW/kg for the total weight of cathode and anode active materials, signifying an important step toward practical application of MXene materials in energy storage devices.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 51677182), the Beijing Municipal Science and Technology Commission (No. Z181100000118006) and the Beijing Nova Program (No. Z171100001117073).
| [1] |
Y. Ma, H. Chang, M. Zhang, Y. Chen, Adv. Mater. 27 (2015) 5296-5308. DOI:10.1002/adma.201501622 |
| [2] |
J. Jiang, P. Nie, S. Fang, et al., Chin. Chem. Lett. 29 (2018) 624-628. DOI:10.1016/j.cclet.2018.01.029 |
| [3] |
B. Li, J. Zheng, H. Zhang, et al., Adv. Mater 30 (2018) e1705670. DOI:10.1002/adma.201705670 |
| [4] |
H. Wang, C. Zhu, D. Chao, et al., Adv. Mater 29 (2017) 1702093. DOI:10.1002/adma.201702093 |
| [5] |
Y. Liu, P. Zhang, N. Sun, et al., Adv. Mater 30 (2018) 1707334. DOI:10.1002/adma.201707334 |
| [6] |
C. Li, X. Zhang, C.K. Sun, et al., J. Phys. D:Appl. Phys 52 (2019) 143001. DOI:10.1088/1361-6463/aaff3a |
| [7] |
F. Yao, D.T. Pham, Y.H. Lee, ChemSusChem 8 (2015) 2284-2311. DOI:10.1002/cssc.201403490 |
| [8] |
K. Naoi, S. Ishimoto, J.I. Miyamoto, W Naoi, Energy Environ. Sci 5 (2012) 9363. DOI:10.1039/c2ee21675b |
| [9] |
C. Li, X. Zhang, K. Wang, et al., Adv. Mater 29 (2017) 1604690. DOI:10.1002/adma.201604690 |
| [10] |
G. Amatucci, F. Badway, A. Pasquier, T Zheng, J. Electrochem. Soc 148 (2001) A930-A939. DOI:10.1149/1.1383553 |
| [11] |
A. Jain, V. Aravindan, S. Jayaraman, et al., Sci. Rep 3 (2013) 3002. DOI:10.1038/srep03002 |
| [12] |
Y. Wang, Z. Hong, M. Wei, Y. Xia, Adv. Func. Mater. 22 (2012) 5185-5193. DOI:10.1002/adfm.201200766 |
| [13] |
Z. Chen, V. Augustyn, J. Wen, et al., Adv. Mater. 23 (2011) 791-795. DOI:10.1002/adma.201003658 |
| [14] |
H. Kim, M. Cho, M. Kim, et al., Adv. Energy Mater. 3 (2013) 1500-1506. DOI:10.1002/aenm.201300467 |
| [15] |
E. Lim, C. Jo, H. Kim, et al., ACS Nano 9 (2015) 7497-7505. DOI:10.1021/acsnano.5b02601 |
| [16] |
Y. Fang, R. Hu, B. Liu, et al., J. Mater. Chem. A 7 (2019) 5363-5372. DOI:10.1039/C8TA12069B |
| [17] |
S. Sun, C. Liao, A. Hafez, et al., Chem. Eng. J. 338 (2018) 27-45. DOI:10.1016/j.cej.2017.12.155 |
| [18] |
M. Naguib, J. Come, B. Dyatkin, et al., Electrochem. Commun. 16 (2012) 61-64. DOI:10.1016/j.elecom.2012.01.002 |
| [19] |
D. Xiong, X. Li, Z. Bai, S Lu, Small 14 (2018) e1703419. DOI:10.1002/smll.201703419 |
| [20] |
M. Xu, N. Bai, H. Li, et al., Chin. Chem. Lett. 29 (2018) 1313-1316. DOI:10.1016/j.cclet.2018.04.023 |
| [21] |
Q. Zhao, Q. Zhu, J. Miao, et al., Nanoscale 11 (2019) 8442-8448. DOI:10.1039/C8NR09653H |
| [22] |
M. Okubo, A. Sugahara, S. Kajiyama, A. Yamada, Acc. Chem. Res. 51 (2018) 591-599. DOI:10.1021/acs.accounts.7b00481 |
| [23] |
J. Shi, Q. Ji, Z. Liu, Y. Zhang, Adv. Energy Mater 6 (2016) 1600459. DOI:10.1002/aenm.201600459 |
| [24] |
L. Yu, L. Hu, B. Anasori, et al., ACS Energy Lett. 3 (2018) 1597-1603. DOI:10.1021/acsenergylett.8b00718 |
| [25] |
O. Mashtalir, M.R. Lukatskaya, M.Q. Zhao, et al., Adv. Mater. 27 (2015) 3501-3506. DOI:10.1002/adma.201500604 |
| [26] |
C. Zhang, S. Kim, M. Ghidiu, et al., Adv. Funct. Mater. 26 (2016) 4143-4151. DOI:10.1002/adfm.201600682 |
| [27] |
J. Halim, S. Kota, M. Lukatskaya, et al., Adv. Funct. Mater. 26 (2016) 3118-3127. DOI:10.1002/adfm.201505328 |
| [28] |
A. Byeon, A. Glushenkov, B. Anasori, et al., J. Power Sources 326 (2016) 686-694. DOI:10.1016/j.jpowsour.2016.03.066 |
| [29] |
J. Luo, W. Zhang, H. Yuan, et al., ACS Nano 11 (2017) 2459-2469. DOI:10.1021/acsnano.6b07668 |
| [30] |
S. Li, T. Wang, Y. Huang, et al., ACS Appl. Mater. Interfaces 11 (2019) 24114-24121. DOI:10.1021/acsami.9b06351 |
| [31] |
J. Zhang, J. Wang, Z. Shi, Z. Xu, Chin. Chem. Lett. 29 (2018) 620-623. DOI:10.1016/j.cclet.2018.01.031 |
| [32] |
K. Zhu, Y. Jin, F. Du, et al., J. Energy. Chem. 31 (2019) 11-18. DOI:10.1016/j.jechem.2018.03.010 |
| [33] |
G. Wang, L. Zhang, J. Zhang, Chem. Soc. Rev. 41 (2012) 797-828. DOI:10.1039/C1CS15060J |
| [34] |
Q. Lu, J. Chen, J. Xiao, Angew. Chem. Int. Ed. 52 (2013) 1882-1889. DOI:10.1002/anie.201203201 |
| [35] |
M. Palacin, Chem. Soc. Rev. 38 (2009) 2565-2575. DOI:10.1039/b820555h |
| [36] |
C. Li, X. Zhang, K. Wang, et al., Carbon 140 (2018) 237-248. DOI:10.1016/j.carbon.2018.08.044 |
| [37] |
X. Zhang, Y. Zhong, Y Yan, Phys. Status Solidi-A 215 (2018) 800014. |
| [38] |
L. Huan, Z. Xin, Z. Yifan, et al., Nano-Micro Lett 11 (2019) 65. DOI:10.1007/s40820-019-0296-7 |
| [39] |
F. Ming, H. Liang, W. Zhang, et al., Nano Energy 62 (2019) 853-860. DOI:10.1016/j.nanoen.2019.06.013 |
| [40] |
Y. Luan, H. Rong, Y. Fang, et al., Nano-Micro Lett 11 (2019) 30. DOI:10.1007/s40820-019-0260-6 |
| [41] |
P. Lian, Y. Dong, Z. Wu, et al., Nano Energy 40 (2017) 1-8. DOI:10.1016/j.nanoen.2017.08.002 |
| [42] |
H. Li, Z. Wang, L. Chen, X. Huang, Adv. Mater. 21 (2009) 4593-4607. DOI:10.1002/adma.200901710 |
| [43] |
J. Luo, J. Zheng, J. Nai, et al., Adv. Funct. Mater 29 (2019) 1808107. DOI:10.1002/adfm.201808107 |
| [44] |
J. Zhou, S. Lin, Y. Huang, et al., Chem. Eng. J. 373 (2019) 203-212. DOI:10.1016/j.cej.2019.05.037 |
| [45] |
D. Sun, M. Wang, Z. Li, et al., Electrochem. Commun. 47 (2014) 80-83. DOI:10.1016/j.elecom.2014.07.026 |
| [46] |
B.H. Deng, T.Y. Lei, W.H. Zhu, et al., Adv. Func. Mater 28 (2018) 1704330. DOI:10.1002/adfm.201704330 |
| [47] |
C. Li, X. Zhang, K. Wang, et al., J. Power Sources 400 (2018) 468-477. DOI:10.1016/j.jpowsour.2018.08.013 |
| [48] |
S. Fletcher, V.J. Black, I. Kirkpatrick, J. Solid State Electrochem. 18 (2013) 1377-1387. |
| [49] |
X. Zhang, X. Sun, H. Zhang, et al., Electrochim. Acta 87 (2013) 637-644. DOI:10.1016/j.electacta.2012.10.022 |
| [50] |
K. Xu, Chem. Rev. 114 (2014) 11503-11618. DOI:10.1021/cr500003w |
| [51] |
T. Kim, G. Jung, S. Yoo, et al., ACS Nano 7 (2013) 6899-6905. DOI:10.1021/nn402077v |
| [52] |
J. Come, M. Naguib, P. Rozier, et al., J. Electrochem. Soc 159 (2012) A1368-A1373. DOI:10.1149/2.003208jes |
| [53] |
X.Q. Han, P.X. Han, J.H. Yao, et al., Electrochim. Acta 196 (2016) 603-610. DOI:10.1016/j.electacta.2016.02.185 |
| [54] |
H.S. Li, L.F. Shen, J. Wang, et al., J. Mater. Chem. A 3 (2015) 16785-16790. DOI:10.1039/C5TA02929E |
| [55] |
P. Yu, G. Cao, S. Yi, et al., Nanoscale 10 (2018) 5906-5913. DOI:10.1039/C8NR00380G |
| [56] |
M.Q. Zhao, C.E. Ren, Z. Ling, et al., Adv. Mater. 27 (2015) 339-345. DOI:10.1002/adma.201404140 |
| [57] |
Y. Dall'Agnese, P. Rozier, P.L. Taberna, et al., J. Power Sources 306 (2016) 510-515. DOI:10.1016/j.jpowsour.2015.12.036 |
 2020, Vol. 31
2020, Vol. 31 

