b Teachers Education College of Harbin Normal University, Harbin 150001, China;
c Key Laboratory of Advanced Chemical Power Sources, Guizhou Meiling Power Sources Co., Ltd., Zunyi 563003, China
Supercapacitors have been regarded as one of promising representative energy storage devices with high-power density due to their unique advantages such as a long cycle life and environmental friendliness [1]. Usually, supercapacitors are classified into two categories based on charge storage mechanism of electrode materials. One is typical electrical double-layer capacitors (EDLCs), which storages charges through adsorbing/ desorbing process at the electrode/electrolyte interface [2]. Although EDLCs provide a stable cycling performance, their low capacitance
cannot meet increasing requirement of high-energy devices. Pseudocapacitors, as another type supercapacitor, permit fast redox reaction on materials surface layer, engendering larger energy density due to more charges transfer [3]. Thus, pseudo-capacitors become a promising candidate that can offer not only high power density but also capable energy density for power equipment. However, traditional pseudocapacitors electrode materials such as RuO2 [4], MnO2 [5-9], V2O5 [10, 11] are confronted with many problems such as high price, low electrical conductivity, and poor cycling performance [12]. Thus, it is necessary to explore capable electrode materials for pseudocapa-citors.
MXene, an emerging group of two-dimensional (2D) materials, have present their potential as electrochemical energy storage materials with high energy and power densities due to the large surface to volume ratios, high conductivity, rich exposed active site, and lack of bulk phase diffusion [13, 14]. Usually, MXene is prepared by selective etching of the most lively "A" atoms layer from the layered transition metal carbides (MAX) by using the synthetic hydrofluoric acid solution (LiF and HCl), which results in abundant of surface functional groups (—OH, —F and —O) [15, 16]. The surface functional groups provide hydrophilicity and a large number of active sites [17]. Recently, Gogotsi's group demonstrated the pseudocapacitance charge storage mechanism of MXene in H2SO4 electrolyte. The Ti oxidation state and deprotonation/ protonation of —O function groups change with the stripping/ plating processes of H+, leading to a large capacitance [2, 18]. However, similar to other 2D materials, when MXene nanosheets are fabricated into electrodes, they suffer from the self-restacking problem due to van der Waals force between layers, resulting in a covered reaction sites and tortuous ions transmission path [15]. Recently, constructing a 3D electrode becomes an effective strategy to enhance the electrochemical performance [19]. R. Lukatskaya et al. synthesized a macroporous MXene film by using polymethyl methacrylate as a template to construct a 3D film electrode, which deliver up to 310 F/g [2]. Xia et al. realized the vertical alignment of MXene by mechanically shearing of a discotic lamellar liquid-crystal phase of Ti3C2Tx and a capacitance of 275 F/g was obtained [13]. These strategies realized short vertical transmission paths, resulting in a favorable rate performance. Unfortunately, the complex and demanding preparation processes limit their large-scale production and application.
Herein, we designed and prepared a porous MXene-RGO flexible film by a self-puffing reaction of MXene-GO film. The sparking reaction of GO created a large amount of cross-linked porous structure between the MXene layers. As-prepared MXeneRGO film was employed as electrode directly without addition of heavy metal current collector and inactive binder. RGO successfully prevents the self-restacking of MXene and the chamber structure between layers can accommodate a little of electrolyte, which causes a close-fitting reaction, avoiding complex ions transmission paths and promising fast ion storage behaviors. Therefore, the MXene-RGO films displayed a large capacitance, remarkable rate ability and stable cycling performance. Moreover, such a unique chamber structure made the capacitance was less affected by thickness of electrode and loading of active materials.
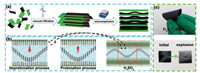
Fig. 1a displays schematic illustration of fabrication process of the Ti3C2Tx-RGO film with an interlayer link-cross porous structure. Firstly, the uniform mixture of few-layered Ti3C2Tx and GO nanosheets was transformed into the compact Ti3C2Tx-GO film through vacuum filtration. Then, Ti3C2Tx-GO film touched a hottable and a micro-explosion reaction occurred. Due to abrupt and larger heat, GO was reduced into RGO. During oxygen-containing groups removal process, generated gas enlarged the interlayer spacing and created a cross-linked porous structure [20]. Finally, Ti3C2Tx-RGO flexible film was obtained. In such a porous structure, H2SO4 electrolyte will infuse the nano-chambers among layers due to the hydrophilic surface of Ti3C2Tx as shown in Fig. 1b [21]. During the discharging and charging electrochemical reaction, the H+ will move toward Ti3C2Tx surface/interlayer, and back into the chamber, respectively. Such a close-fitting reaction can accelerate the ions transport. Different with the lengthy and tortuous H+ transmission path in traditional multilayer Ti3C2Tx films, such reaction process achieves ultra-fast ions storage behavior. In addition, the porous structure prepared by micro-explosion makes Ti3C2Tx separate from each other, which enables single layer or a few layers of Ti3C2Tx participate in the reaction at each nanoelectrode between the layers, increasing electrochemical utilization. Fig. 1c shows the images of Ti3C2Tx-GO and Ti3C2Tx-RGO films. Ti3C2Tx-GO film shows a black and smooth surface. After micro-explosion, the Ti3C2Tx-RGO film presented gray and pleated surface due to reducing process and increased interlayer spacing. Meanwhile, Ti3C2Tx-RGO film displayed a good flexibility.
|
Download:
|
| Fig. 1. (a) Synthetic schematic for Ti3C2Tx-RGO film; (b) Reaction mechanism for Ti3C2Tx-RGO in H2SO4 electrolyte; (c) Physical pictures for Ti3C2Tx-GO and Ti3C2Tx-RGO films. | |
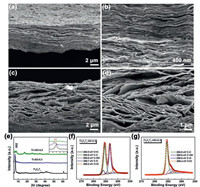
The microscopic morphologies of the Ti3C2Tx-GO and Ti3C2TxRGO films were observed by a scanning electron microscopy (SEM). Figs. 2a and b exhibit a tightly stacked structure for Ti3C2Tx-GO film, and Figs. 2c and d display a cross-linked and porous structure in the Ti3C2Tx-RGO interlayer after micro-explosion. It is noteworthy that the layer spacing is enlarged and the chamber height ranges from 100 nm to 1 μm, implying that a few of electrolyte can be stored between layers. X-ray diffractometer (XRD) was employed to investigate crystal structure changes from Ti3AlC2 to Ti3C2Tx and from Ti3C2Tx-GO to Ti3C2Tx-RGO. As shown in Fig. S1 (Supporting information), the highest peak at 39.5° of Ti3AlC2 was completely obliterated and the (002) characteristic peak of Ti3C2Tx before 10° occurred after etching and ultrasound process, suggesting successful synthesis of Ti3C2Tx MXene [3]. For the Ti3C2Tx-GO film, the (002) peak strength weakened and moved 0.6° to the left due to the introduction of GO and increased interlayer spacing, which is similar to Yan's report [15]. After the reduction process, (002) peak of the Ti3C2Tx-RGO film became very wide and flat, which can be attributed to enlarged layer spacings and different orientations for Ti3C2Tx nanosheets (Fig. 2e) [22]. In order to investigate the functional groups on the film surface before and after the micro-explosion, X-ray photoemission spectroscopy (XPS) was performed. The C 1s spectra as shown in Fig. 2f indicated the existence of lots of oxygen-containing groups on Ti3C2Tx-GO film, with the peaks at 284.0, 284.6, 285.6, 286.7 and 288.2 eV correspond to C = C, C——C, C——OH, C–O and C=O bonds, respectively [23]. After the micro-explosion, a large proportion of function groups were removed, which can be attributed to the reduction process from GO to RGO (Fig. 2g). In contrast, from Ti 2p (Fig. S2 in Supporting information) and F 1s (Fig. S3 in Supporting information) spectra, Ti3C2Tx surface functional groups had almost no change, suggesting stable functional groups and stronger binding energy between Ti3C2 and Tx. The generated RGO enhance electronic conductivity and remained functional groups ensure the hydrophilicity, promising the fast penetration of electrolyte.
|
Download:
|
| Fig. 2. (a–d) Microscopic morphology and (e) XRD before and after micro-explosion reaction of Ti3C2Tx-GO films; XPS for (f) Ti3C2Tx-GO and (g) Ti3C2Tx-RGO. | |
Electrochemical performance of Ti3C2Tx-RGO freestanding electrode was evaluated in a three-electrode system with carbon-point as a counter electrode and Ag/AgCl as a reference electrode in 3 mol/L H2SO4 electrolyte. The detailed experimental method is provided in supporting information. To investigate the synergy effect between Ti3C2Tx and RGO, and their optimized mass ratio, five samples were designed and prepared with the same quality (Table 1).
|
|
Table 1 Samples composition and number of Ti3C2Tx-RGO films. |
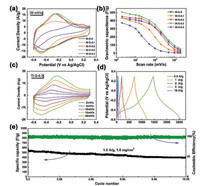
Fig. 3a shows the cyclic voltammetry (CV) curves of pure Ti3C2Tx and Ti3C2Tx-RGO films at the scan rate of 20 mV/s. When the content of GO is little, Ti3C2Tx-RGO-0.1 films exhibit smaller integral area, which can be attributed to a smaller layer spacing variation. When the content of GO is nimiety, the smallest area was obtained for Ti3C2Tx-RGO-0.4 without redox peaks, due to low capacitance and conductivity of RGO. In contrast, Ti3C2Tx-RGO-0.2 and Ti3C2Tx-RGO-0.3 films display larger area than the pure Ti3C2Tx, and a couple of redox peaks locate at —0.3 V and —0.45 V (vs. Ag/AgCl) represent a reversible pseudocapacitance reaction associated with the deprotonation/protonation process of functional groups and the change of Ti oxidation state. The CV curves of these at different scan rates are shown in Fig. 3c and Fig. S4 (Supporting information). Ti3C2Tx-RGO-0.3 film kept the shape best, suggesting the outstanding rate ability. The specific capacitance was calculated from the CV curves as shown in Fig. 3b. At 2 mV/s, the Ti3C2Tx-RGO-0.3 showed the highest capacity of 505 F/g (808 F/cm2), among Ti3C2Tx-RGO-0.2 (452 F/g), Ti3C2Tx (436 F/g), Ti3C2Tx-RGO-0.1 (433 F/g) and Ti3C2Tx-RGO-0.4 (373 F/g) samples. Even at 100 mV/s, Ti3C2Tx-RGO-0.3 still maintained a high capacity of 353 F/g (564.8 F/cm2). It suggests that the optimal ratio of Ti3C2Tx:GO is 7:3. It should be noticed that the gravimetric capacitance of 505 F/g (561 C/g in the voltage window of 0.9 V) is closed to its theoretical value (615 C/g) reported by Yury's group [2]. Such a result is competitive with previous excellent reports is shown in Table S1 (Supporting information). Ti3C2Tx-RGO-0.3 film exhibits the nonlinear galvanostatic charge/discharge curves with a couple of charge/discharge platforms corresponding to redox peaks in the CV curves (Fig. 3d). The discharge specific capacitance was calculated to be up to 612 F/g at 0.5 A/g, followed by 505, 386, 385 and 371 F/g at 1.0, 2.0, 5.0 and 10 A/g, respectively. In addition, Ti3C2Tx-RGO-0.3 presents an excellent cycling performance at 1.0 A/g as shown in Fig. 3e, corresponding to the decay of only 0.0071% per cycle. In order to certify the practical application value, an asymmetric supercapacitor was assembled with the Ti3C2Tx-RGO- 0.3 as a cathode and active carbon (AC) as an anode. The electrochemical performance was performed in 0~ 1.0 V as shown the Fig. S5 (Supporting information). Stable CV curves indicate a good dynamic match for cathode and anode. A pair of slight redox peaks (0.6 V and 0.5 V) can be observed and classified as a Faraday reaction of Ti3C2Tx.
|
Download:
|
| Fig. 3. Electrochemical performance for Ti3C2Tx-RGO films in 3 mol/L H2SO4 electrolyte. (a) CV curves at 20 mV/s and (b) gravimetric capacitances at different scan rates of different Ti3C2Tx-RGO films; (c) CV curves, (d) charge-discharge curves and (e) cycling performance of Ti3C2Tx-RGO-0.3 film. | |
To further understand electrochemical dynamics of Ti3C2Tx-RGO-0.3 film, the mechanism of electrochemical process was investigated. In the CV curves, the peak current (i) and scan rate (v) obey the relationship as follow:
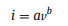
|
(1) |
in which the a and b are the constant and b-value is between 0.5 to 1.0. When the electrochemical process controlled by diffusion behavior, the b-value is close to 0.5, while the b-value of 1.0 represents a fast reaction without diffusion-controlled [24, 25]. As shown in Fig. 4a, the b-values of Ti3C2Tx-RGO-0.3 electrode are 0.86 and 0.93 for cathodic and anodic peaks, respectively. In contrast, the lower b-values of 0.84 and 0.89 of pure Ti3C2Tx film as shown in Fig. 4d indicate a slower reaction rate due to the longer transmission path of H+ in the tightly stacked layers. While the modified electrode structure makes a close-fitting electrochemical reaction, that is, interlayer MXene reacts directly with H+ in the interlayer pores, avoiding longer transport. In order to further certify that, the impedance spectroscopy of they were studied through a complex model capacitance as follow:

|
(2) |
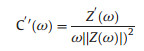
|
(3) |
in which, the z'(ω), z"(ω), and ω represent real, imaginary parts, and angular frequency of the complex impedance, respectively; while the C'(ω) and C"(ω) are the real and imaginary parts for the complex capacitances [24]. As shown in Figs. 4b and e, the C'(ω) decreases slower with the frequency for Ti3C2Tx-RGO-0.3 electrode, suggesting faster diffusion and reaction rate of ions. In addition, the minimal characteristic relaxation time constant t0 (the minimum time required to release all energy when the efficiency is greater than 50%) can be calculated by the characteristic frequency f0, corresponding to the half value of C' and the highest value of C" [15]. Half of the time (3.3 s) of MXeneRGO-0.3 electrodes was obtained compared to the pure MXene (6.6 s). The above results demonstrate the faster reaction dynamics for the porous MXene-RGO-0.3 film. Moreover, on account of the uniform intralayer structure, Ti3C2Tx-RGO film will get rid of the surface loading limit, which is an affliction in traditional tight stacked film. As shown in Fig. 4f, the specific capacitance of pure Ti3C2Tx film decreases sharply with increasing surface loading. In contrast, Ti3C2Tx-RGO-0.3 presents a good rate performance independent of the mass loading (Fig. 4c), which is very industrially significant for obtaining high-energy electrochemical devices.

|
Download:
|
| Fig. 4. Performance comparison between Ti3C2Tx-RGO-0.3 (up) and pure Ti3C2Tx films (below). (a, d) The relationship between peak currents and scan rates from 2 mV/s to 50 mV/s; (b, e) Normalized real and imaginary capacitances; (c, f) Gravimetric capacitances of different surface loads in at different scan rates. | |
In summary, a flexible cross-linked porous Ti3C2Tx-RGO film was prepared through a micro-explosion reaction, which prevented the self-restacking of MXene, increased interlayer spacing obviously and exposed a large number of electrochemical active sites. Furthermore, the chamber structure between layer could accommodate a few of electrolyte, achieving a close-fitting reaction and avoiding lengthy transmission paths of active ions. As-prepared Ti3C2Tx-RGO-0.3 film exhibited a high rate performance and ultra-high gravimetric capacitance of 505 F/g at 2 mV/s and 353 F/g at 100 mV/s in the 3 mol/L H2SO4 electrolyte. Meanwhile, it also displayed excellent cycling stability. In addition, the unique close-fitting reaction and uniform intralayer structure promised the electrochemical performance of Ti3C2Tx-RGO film is less effect by mass loading than pure Ti3C2Tx film. This work paved a way for the design and development of capable electrochemical energy storage devices with high mass loading of active materials.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51702063, 51672056), Natural Science Foundation of Heilongjiang Province (No. LC2018004), China Postdoctoral Science Foundation (Nos. 2018M630340, 2019T120254) and the Fundamental Research Funds for the Central University (No. 3072019CF1006). The authors also thank the support from the Starting Research Fund from Harbin Normal University (No. XKB201420).
Appendix A. Supplementary dataSupplementary material related tothis article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.08.043.
| [1] |
Y. Wang, Y. Song, Y. Xia, Chem. Soc. Rev. 45 (2016) 5925-5950. DOI:10.1039/C5CS00580A |
| [2] |
M.R. Lukatskaya, S. Kota, Z. Lin, et al., Nat. Energy 2 (2017) 17105. DOI:10.1038/nenergy.2017.105 |
| [3] |
M. Boota, Y. Gogotsi, Adv. Energy Mat 9 (2019) 1802917. DOI:10.1002/aenm.201802917 |
| [4] |
Q. Jiang, N. Kurra, M. Alhabeb, Y. Gogotsi, H.N. Alshareef, Adv. Energy Mater 8 (2018) 1703043. DOI:10.1002/aenm.201703043 |
| [5] |
X. Zhang, P. Yu, H. Zhang, et al., Electrochim. Acta 89 (2013) 523-529. DOI:10.1016/j.electacta.2012.11.089 |
| [6] |
X. Zhang, X. Sun, H. Zhang, D. Zhang, Y. Ma, Electrochim. Acta 87 (2013) 637-644. DOI:10.1016/j.electacta.2012.10.022 |
| [7] |
X. Zhang, X. Sun, H. Zhang, C. Li, Y. Ma, Electrochim. Acta 132 (2014) 315-322. DOI:10.1016/j.electacta.2014.03.176 |
| [8] |
J. Qin, Z.S. Wu, F. Zhou, et al., Chin. Chem. Lett. 29 (2018) 582-586. DOI:10.1016/j.cclet.2017.08.007 |
| [9] |
Y. Chen, C. Chen, R. Lv, et al., Chin. Chem. Lett. 29 (2018) 616-619. |
| [10] |
F. Liu, Z. Chen, G. Fang, et al., Nano-Micro Lett 11 (2019) 25. DOI:10.1007/s40820-019-0256-2 |
| [11] |
Y. Yang, Y. Tang, G. Fang, et al., Energy Environ. Sci. 11 (2018) 3157-3162. DOI:10.1039/C8EE01651H |
| [12] |
C. Wang, P. Sun, G. Qu, J. Yin, X. Xu, Chin. Chem. Lett. 29 (2018) 1731-1740. DOI:10.1016/j.cclet.2018.12.005 |
| [13] |
Y. Xia, T.S. Mathis, M.Q. Zhao, et al., Nature 557 (2018) 409-412. DOI:10.1038/s41586-018-0109-z |
| [14] |
K. Zhu, Y. Jin, F. Du, et al., J. Energy Chem. 31 (2019) 11-18. DOI:10.1016/j.jechem.2018.03.010 |
| [15] |
J. Yan, C.E. Ren, K. Maleski, et al., Adv. Funct. Mater 27 (2017) 1701264. DOI:10.1002/adfm.201701264 |
| [16] |
Q. Shan, X. Mu, M. Alhabeb, et al., Electrochem. Commun. 96 (2018) 103-107. DOI:10.1016/j.elecom.2018.10.012 |
| [17] |
S. Zhao, X. Meng, K. Zhu, et al., Energy Storage Mater. 8 (2017) 42-48. DOI:10.1016/j.ensm.2017.03.012 |
| [18] |
M. Hu, Z. Li, T. Hu, et al., ACS Nano 10 (2016) 11344-11350. DOI:10.1021/acsnano.6b06597 |
| [19] |
X. Liu, R. Zhang, W. Yu, et al., Energy Storage Mater. 11 (2018) 83-90. DOI:10.1016/j.ensm.2017.09.008 |
| [20] |
D. Lin, Y. Liu, Z. Liang, et al., Nat. Nanotechnol 11 (2016) 626. DOI:10.1038/nnano.2016.32 |
| [21] |
Z. Ma, K. Wang, Y. Qiu, et al., Energy 143 (2018) 43-55. DOI:10.1016/j.energy.2017.10.110 |
| [22] |
M.Q. Zhao, X. Xie, C.E. Ren, et al., Adv. Mater 29 (2017) 1702410. DOI:10.1002/adma.201702410 |
| [23] |
H. Hu, Z. Zhao, W. Wan, Y. Gogotsi, J. Qiu, Adv. Mater. 25 (2013) 2219-2223. DOI:10.1002/adma.201204530 |
| [24] |
Y. Fang, R. Hu, B. Liu, et al., J. Mater. Chem. 7 (2019) 5363-5372. |
| [25] |
Y. Luan, R. Hu, Y. Fang, et al., Nano-Micro Lett 11 (2019) 30. DOI:10.1007/s40820-019-0260-6 |
 2020, Vol. 31
2020, Vol. 31 


