b State Key Laboratory of Organic-Inorganic Composites, Beijing Key Laboratory of Electrochemical Process and Technology for Materials, Beijing University of Chemical Technology, Beijing 100029, China;
c National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro 76080, Pakistan
MXenes, a recently introduced family of 2D materials from Drexel University, is already gaining paramount attention in the scientific community. In general, MXenes are 2D transition metal carbides, nitrides, or carbonitrides with a representative formula of Mn+1XnTx. Here M can be a transition metal, X can be carbon (C) or nitride (N), n = 1~3, and Tx represents the associated functionality. MXenes could easily be acquired by selective etching of the "A"layer from the MAX phase, i.e., an element from groups 13–14 of the periodic table, using hydrofluoric acid or a mixture of lithium fluoride and hydrochloric acid [1]. At present, among the many theoretically predicated MXenes, the Ti3C2, Ti2C, Nb2C, V2C, Ti3CN, Mo2C and Ta4C3 members have been successfully synthesized [2]. Amongst them, Ti3C2Txis one of the most common and widely studied MXene [3, 4]. MXenes are already showing tremendous potential in energy applications, mainly as electrode materials in batteries and supercapacitors [5-7]. The versatile surface chemistry, graphene-like morphology, and redox capability with metal-like conductivity render MXenes as a suitable 2D-material for diverse applications. Since MXenes are composed of non-toxic yet abundant elements suchasTi, C and N with their degraded products(CO2andN2), which are also non-toxic, MXenes could also be utilized for environmental applications [8]. Apart from MXenes' energy-related applications [6, 9], these 2D materials are slowly gaining attention from the sensor community. The high surface conductivity, adequate surface terminated functionality and the most important of all excellent hydrophilicity recognize MXenes as an ideal platform to develop different sensor devices. Mainly, in electrochemical sensor systems, where transducing material plays a crucial role in producing measurable signal readout that could either be directlyor indirectly related to the concentration of target species. Unlike the conventional 2D materials, i.e., graphene, MoS2 nanosheets, which require complex synthesis protocols and possess low-hydrophilicity with difficult functionalization approach, MXenes offer superiority in all aspects including biocompatibility which is preferred in case of biosensors. There are only a few review papers that provide a concise overview discussing the use of MXenes in sensory systems at present. Zhu et al. [10] focused on the applications of MXenes in catalysis, sensors and adsorption in his review. Similarly, Sinha et al. [11] complied literature dealing with the use of MXenes in different types of sensor systems. However, these reports mainly concern with the chemical diversity and superiority of MXenes. The present short review aims to discuss the critical aspects while defining MXenes' pros and cons when considered for electro-chemical driven sensory techniques.
Here, we first discuss the need of MXenes for the sensors followed by their use as a direct transducing material or as a substrate for immobilization of recognition element in the case of biosensors. Based on the nature of the acquired signal, this short review is sub-divided into three sections discussing the most recent MXenes driven up-gradation in electrochemical, gas and electro-chemiluminescence & fluorescent sensors. The suitability of MXenes in modifying electrodes with their analytical capability to detect different species is discussed, and endeavors have been made to highlight the future perspective of MXenes in advance sensor devices.
2. Why MXenes for sensors?The field of sensors has evolved unprecedentedly following the integration of advanced nanomaterials. This integration has expended the application of sensors beyond the detection of simple bio-molecules like glucose [12]. The recent advancement in sensory devices in terms of engineering and configuration has realized their potential application further into the field of biomedical sciences, therapeutics, environment, pharmaceuticals, and food science [13]. Amongst all sensor devices, the basic configuration involves a working electrode (WE) that measures potential against a reference (RE) and a counter electrode (CE). This WE could easily be engineered with different nanomaterials to construct different configurations capable of detecting a variety of species. Thus, electrode engineering is crucial for designing a robust sensor system [14, 15]. In addition, the feasibility of using different techniques for target recognition and signal transduction has empowered sensor devices to stipulate according to the nature and matrix environment of the target species. When it comes to signal transduction, the choice of transducer (material) is crucial. In the case of electrochemical sensors, the conventional transducers with their low-surface area suffer from the need of high over-potential besides the slow charge kinetics and mass transfer limitation of the target analyte. The strain sensors, on the other hand, require the materials to maintain conductivity and mechanical strength. Thus, the transducer material for any practical sensor device should possess major characteristics like high conductivity, large surface area, good mechanical strength besides wide availability, and modest synthesis recipe. To achieve this, a variety of materials ranging from metals, metal oxides to polymers and carbonaceous materials with their combinations as composites and hybrids have been explored [16-20]. However, the race to find the best combination with high signal throughput is still on-going. Most recently, Drexel University reported the discovery of a new class of 2D materials known as MXenes [21]. These 2D materials, despite their primary use as energy material, i.e., batteries and supercapacitors, are gaining un-parallel attention from the sensor community [22-25]. The main reason for their widening scope in sensor science is directly related to their versatile surface and chemical characteristics. Unlike graphene, which regardless of its good conductivity, is challenging to integrate with electrode systems for reasons including low hydrophilicity, and folding or re-stacking of sheets, MXenes on the other hand offer relatively high hydrophilicity with abundant surface —OH, —O and/or —F, moieties to anchor other functional groups of choice, i.e., ease of surface functionalization [26]. In addition, the extremely high conductivity of MXenes (850-2410 S/cm) makes them a substrate of choice when it comes to designing an electrochemical sensor [27]. This conductivity, coupled with robust mechanical characteristics also recognizes MXenes as a suitable conductive filler for strain and pressure sensors [28]. The excellent surface characteristics, metal-like conductivity, and flexibility with just right magnitude of stiffness recognizes MXenes' as an attractive transducing material for many kinds of sensors, including electrochemical, bio-electrochemical, strain, pressure and chemiluminescent and photochemical sensors [22]. Fig. 1 represents the doughnut chart depicting the use of MXenes in various types of sensors utilized for a variety of applications. The classification is based on the nature of signal output, i.e., current. The most recognition presently is received in the electrochemical areas, whereas the use of MXenes in biosensors, gas, and electro-chemiluminescence and fluorescent sensors is gradually increasing. Here, MXenes and their compositional counterparts with the ease of synthesis, metallic nature, wide availability, tunable surface properties, adequate flexibility, and biocompatibility are well-positioned to improve the inherent characteristics of sensor devices.

|
Download:
|
| Fig. 1. Diagram representing applications of MXenes in various kinds of sensor systems with sub-tiles indicating the area of applications of those sensors. | |
3. Application of MXenes in electrochemical (bio) sensors
MXenes with their intrinsic properties such as conductivity, high surface area and thin-flake like morphological features have been utilized in electrochemical sensors and biosensors whose primarily signal response is related to the electrode interface. The high conductivity, coupled with the redox characteristics of MXenes, i.e., Ti3C2Tx enables it to simultaneously act not only as a charge-transfer facilitator but also as a catalyst to participate in the electrochemical oxidation or reduction reactions.
In this section, we highlight the electrochemical and bioelectrochemical sensors which utilize MXenes and their hybrids as direct or indirect redox transducer and as an electro-catalyst. The use of MXene (Ti3C2Tx) for direct electrochemical sensing of carbendazim pesticide was reported by D. Wu et al. [29]. Wu and his co-workers demonstrated that MXenes owing to their surface functionalities could directly be used as an electro-catalyst to oxidize carbendazim. Although Ti3C2Tx, in most cases, is thought to oxidize above 600 mV, in this case, Ti3C2Tx generated a stable response, which was argued to be the consequence of the different chemical status of Ti3C2Tx obtained from distinct chemical preparation procedure. The electro-catalytic activity was ascribed to the presence of functional moieties on the surface of Ti3C2Tx, which was also supported by the theoretical study between fluorine and oxygen terminated MXenes. The obtained data indicate greater interactions of carbendazim with fluorine terminated MXenes, whereas the electronic properties evaluated using DOS calculation were also in support of increased DOS at the Fermi level, after the adsorption of carbendazim over Ti3C2F2. Thus, this initial assessment provides the evidence of redox activity of MXenes and the influence of surface functionalities on signal output. Despite the excellent conductivity of MXenes, stabilization of their thin layers or sheets during anodic sweep is still a major challenge. At present, surface modification of MXenes seems to be a viable option. In this context, P. Abdul Rasheed et al. [30] demonstrated a unique approach of utilizing the full potential of MXene as electro-catalyst simultaneously using its inherent reduction capability to form in-situ Pd nanoparticles for electrochemical detection of a biomolecule. This process enabled the formation of Pd@Ti3C2Tx composite, where in-situ grown Pd not only contributed to the stabilization of MXene sheets but also reinforced the catalytic activity towards direct electrocatalytic detection of L-cysteine (L-Cys) amino acid. Interestingly, MXene, in this case, plays a dual role of reducing agent, as well as an electrocatalyst. The in-situ grown Pd nanoparticles serve as active centers facilitating fast-charge transportation of electron. This synergic combination generated a stable and improved signal response enabling sensitive amperometric detection of L-Cys in range of 0.1-20 mmol/L with a detection limit of 0.14 μmol/L and signal sensitivity of 5.71 mA L mol-1 cm-2. The developed platform, in this case, was also validated for real biological samples, which further assured the potential application of such composites in clinical applications. In a similar approach, the MXene-MWCNT composite has been considered for the simultaneous sensing of environmental pollutants like catechol (CT) and hydroquinone (HQ). The simultaneous determination of these molecules is complicated, based on their similar chemical and electrochemical behavior. R. Huang et al. [31] taking advantage of highly conductive Ti3C2Tx with MWCNT, demonstrated simultaneous detection of CT and HQ in a linear range of 2 μmol/L to 150 μmol/L. The application of DPV technique enabled a low-detection limit of 6.6 and 3.9 nmol/L for CT and HQ, respectively.
The direct use of MXenes as redox activate surfaces has also been realized in the electrochemical sensing of pharmaceutical drugs. Y. Zhang et al. [32] considered Ti3C2Tx sheets modified screen-printed electrode (SPE) for the oxidation of acetaminophen (ACOP) and isoniazid (INZ) drugs. The direct use of MXene in an acidic electrolyte (0.1 mol/L H2SO4) enabled the linear detection range of 0.25 μmol/L to 2000 μmol/L and 0.1 mmol/L to 4.6 mmol/L with detection sensitivity achievable up to 0.048 μmol/L and 0.064 μmol/L for ACOP and INZ, respectively. In another interesting approach, MXene was used in the reduction-based electrochemical sensing of bromate (BrO3-) ions, taken as a target water pollutant. P. A. Rasheed et al. [33] utilized Ti3C2Tx modified GCE electrode to reduce bromate ions in water, simultaneously generating a reduction signal in a linear concentration range of 50 nmol/L to 5 μmol/L with LOD value of 41 nmol/L. The study highlighted the reduction behavior of MXene, with evidence of the surface changes that occur after the electrochemical reduction of bromate ions. The formation of TiO2 nanoparticles during electrochemical reduction was found to influence the signal sensitivity obtained for bromate ions promptly. The redox capability of MXenes has been deemed most effective against small molecules. For example, MXene has shown tremendous potential in sensing neurotransmitter molecules such as dopamine (DP) [34]. The immobilization of Ti3C2Tx over GCE was facilitated using Nafion, thereby creating a platform highly sensitive towards DP. Interestingly, when compared with the GO-based electrode, the performance of Ti3C2Tx based sensor was found to be far superior with signal sensitivity, mainly relying on the thickness or concentration of the Nafion layer. The performance of Ti3C2Tx based sensor was relatively higher than the one where MXene composite with Pd and Pt is utilized for the detection of DP [35]. In the case of hybrids, L. Lorencova et al. [36] reported the use of Pt modified MXene based sensor for the detection of small molecules. The composite was considered for the oxidation of molecules such as acetaminophen, ascorbic acid, dopamine, uric acid and H2O2. In their study, improved anodic stability of MXenes has been associated with the hybrid configuration, which is responsible for sensitive oxidation signal, particularly for H2O2 [37].
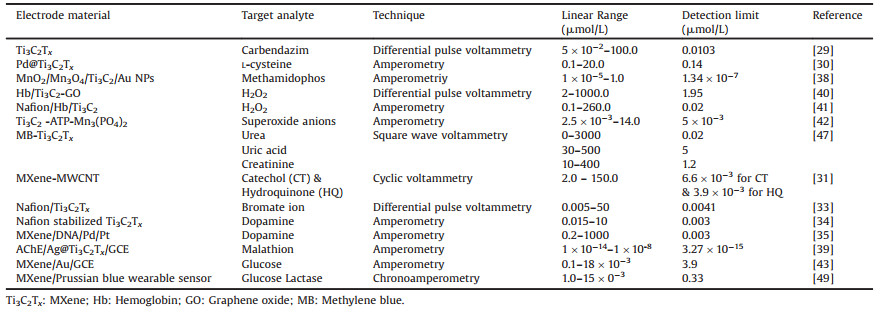
The excellent conductivity, coupled with thin flake-like structural features, infers MXenes to serve as an immobilization platform. Unlike the conventional immobilization platforms where good conductivity is usually compromised over large immobilization surfaces or for abundant catalytic active sites, MXenes have the perquisites to become attractive immobilization platforms. In this context, D. Song et al. [38] reported the use of Au/MXene and MOF-derived MnO2/Mn3O4 as a composite-based platform for the immobilization of acetylcholinesterase (AChE) enzyme. The AChE immobilized platform was later employed to develop a biosensor for detecting methamidophos pesticide (ATCl) based on irreversible inhibition achieved via binding interaction between enzyme and pesticide. Fig. 2 shows the generalized schematic, which describes the step of formation of MOF driven MnO2/Mn3O4 and MXene/Au composites along with the configuration of the developed biosensor. MnO2/Mn3O4 with the MXene/Au NPs configured to consist of abundant active sites, where MXene as a conductive substrate not only supported greater immobilization but also facilitated the interfacial charge transfer process. Apart from improving the stability of MXene sheets, the formed composite, produced a significantly boosted current response for the electro-catalytic oxidation of thiocholine. This boosted current signal was highly sensitive towards methamidophos, whose presence inhibited the obtained current response subsequent to phosphorylation, which decreased the immobilized AChE activity. The approach enabled a highly stable yet responsive signal capable of detection up to 1.34 10-13 mol/L of target pesticide. To further improve the detection sensitivity of AChE based sensors, Y. Jiang et al. [39] developed a unique platform where inherent reduction properties of Ti3C2 were used to produce Ag NPs over its surface (Ag@Ti3C2Tx). The platform then was considered for the immobilization of AChE to probe malathion in the concentration range of 10-14 mol/L to 10-8 mol/L.
|
Download:
|
| Fig. 2. (a) Formation of MnO2/Mn3O4 composite from Mn-MOF precursor. (b) Growth of Au NPs over etched Ti3C2. (c) Illustration for the configuration of a biosensor. Reprint with permission [38]. Copyright 2019, Elsevier Publisher. | |
Among many analytes, the detection of H2O2, in regard to its extensive usage as an oxidizing agent in various industries and as reactive oxygen radial in biological species, has also attracted the attention of MXene-driven sensors. Conventionally, H2O2 could be quantified either enzymatically or non-enzymatically. However, the direct electron-transfer (DET) between the enzyme and the electrode interface is quite sluggish, which results in the loss of the enzyme's bioactivity. Modification of the electrode with an extremely sensitive transducer is one of the most convenient routes to overcome this problem. Since the concentration that is being dealt with in biological samples is very low, high throughput is a necessity to produce measurable signal readout. MXenes and their composites have demonstrated some promising results.Initially, the direct use of MXene for the detection of H2O2 was described by L. Lorencova et al. [37]. In another approach, Zheng et al. [40] showed the remarkable potential of MXene as an immobilization matrix and as a redox mediator to detect H2O2 using hemoglobin (Hb) as an enzyme, immobilized over MXenegraphene oxide (Ti3C2–GO) composite film. The composite was primarily coated over a glass substrate using an ink-jet printer to construct a working electrode with a homogenized surface. The inclusion of GO, not only served as an additional component to exaggerate the active surface area, but also increase the stability of MXene enabling better micro-fabrication. The fabricated electrode, based on its highly conductive surface, generated a robust response for H2O2 at the low-over potential value of 0.08 V. The favorable surface functionality and excellent bio-compatibility of MXene allowed the enzyme to retain its bio-activity even after several repetitive measurements. The sensor response was found logarithmically linear to H2O2 in the concentration range of 2 μmol/L to 1 mmol/L with a LOD value of 1.95 μmol/L. The high throughput signal was basically a synergic effect obtained by coupling MXene and GO, which provided high surface area in addition to the dense active site for catalysis and immobilization of enzyme. The platform was also found suitable for detecting H2O2 within serum samples with high signal selectivity, implying its potential application for clinical purposes. In a similar approach, a mediator-free biosensor for H2O2 was also fabricated by Wang et al. [41] and his team. Here, Hb was used as an enzyme with Ti3C2 as an interfacial transducer and immobilization matrix. Although the approach was sensitive towards H2O2, The measured redox couple indicated fast yet quasi–reversible interfacial electron transfer, which suggested that the redox-active centers could be situated within the protein shell, making the DET relatively difficult. The sensor relied on amperometric technique for the quantification of H2O2 with the linear response obtained in the concentration range of 0.1 μmol/L to 260 μmol/L and LOD value of 20 nmol/L. The use of pristine MXene (Ti3C2Tx) as an immobilization matrix for Hb, revealed 93% response retention after 3 weeks of initial fabrication. In recent work, the use of Ti3C2Tx nanosheets was also considered for anchoring Mn3(PO4)2 NPs, synthesized using ATP as a template to construct a platform for the detection of superoxide anions (O2·-) from HepG2 cells [42]. The Mn3(PO4)2 nanoparticles are known for their catalytic characteristics towards the dismutation of O2·-, making it a suitable electrochemical mediator for the assessment of initial oxidative damage to brain tissue caused by different neurodegenerative diseases. In this case, MXene served as an effective platform to anchor Mn3(PO4)2 NPs, providing effective charge-transfer acceleration and increased signal sensitivity. Taking advantage of the coupled system, J. Zheng et al. [42] and his team were able to detect superoxide anion in the range of 2.5 nmol/L to 14 μmol/L using amperometry as the primary mode of quantification. The signal sensitivity, in this case, was up to 0.5 nmol/L of O2·-. The developed sensor was also capable of quantifying O2·- ions from HepG2 cancer cells, realizing its potential in clinical applications.
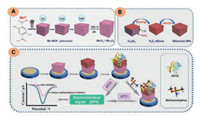
Among biosensor, MXenes have attracted immense attention. Initially, R. B. Rakhi et al. [43] considered Ti3C2Tx for immobilization of glucose oxidase enzyme for enzymatic sensing of glucose. To achieve higher signal sensitivity, Ti3C2Tx was coupled with Au NPs providing the sensor high detection leverage. In this context, L. Wu et al. [44] considered the use of Ti3C2-MXene for the immobilization of tyrosine enzyme. Based on the abundant 2D laminar area, the Ti3C2 could immobilize sufficient concentration of the enzyme. This immobilized platform was then used for the construction of mediator free biosensor for phenol. The oxidation of phenol to o-quinone by tyrosine enzyme generated a signal which was subsequently boosted by reduction of o-quinone to polyhydric phenol. The strategy enabled phenol detection in a range of 0.05 μmol/L to 15.5 μmol/L and LOD value of 12 nmol/L. The aminosilane functionalized MXene has also been considered for the detection of cancer biomarkers. S. Kumar et al. [45] functionalized Ti3C2Tx with aminosilane, which was later used for covalent immobilization of carcinoembryonic (CEA) antibodies. This platform was then used for probing CEA biomarker using ([Ru(NH3)6]3+ as a redox mediator. The sensor exhibited high selectivity towards CEA in the concentration range of 0.0001– 2000 ng/mL with electrode sensitivity of 37.9 μA ng mL-1 cm-2. In a similar context, the capability of MXene in the development of aptasensors has also been investigated. S. Zhou et al. [46] reported the application of Ti3C2Tx coupled with phosphomolybdic acid (PMo12) embedded with polypyrrole (PPy@Ti3C2Tx/PMo12) to detect osteopontin (OPT). OPT is a phosphoprotein responsible for the regulation of tumor metastasis. The dense chemical functionality of PPy@Ti3C2Tx/PMo12 could form G-quadruplex with OPT which allowed its sensitive detection with a lower detection limit of 0.98 fg/mL. The inclusion of MXenes and their hybrids in the field of applicable portable devices such as microfluidic chips and wearable sensors has shown tremendous potential for practical applications. MXene, with its flexibility yet firm laminar structure, could easily be integrated into microfluidic beds and flexible wearable sensors. The concept of engineering MXene in portable devices was experimentally studied by J. Liu et al. [47]. In their study, the multifunctional capabilities of MXene substrate were highlighted using the microfluidic chip as a model device. This device was further used in continuous monitoring of whole blood for hemodialysis, uric acid, urea, and creatinine. Here, MXene was not only used for the immobilization of enzymes but also acted as a substrate to anchor MB to produce an independent signal for ratiometric evaluation. This approach not only provided a sensitive signal, but also minimized the signal drifting and fouling issues of the electrode. The detection of each analyte was based on a different mechanism. In the case of uric acid (UA), inhibition strategy was considered. Initially, UA adsorbed onto the surface of MXene subsequent to the formation of hydrogen bonds between UA and functional moieties of MXene (i.e., OH, O and F). The inhibition in measured current response for the electro-catalytic oxidation of UA in reference to the probe current (MB) was then measured as the primary signal directly proportional to the concentration of UA (Fig. 3a). In the case of urea, a unique approach was designed. Since the by-product of urea catalysis is hydroxyl ions (i.e., pH), the catalysis of urea can directly alter the pH of the system, which in turn can affect the electro-catalytic oxidation signal of UA without changing the signal response of MB. Thus, using this approach, urea was detected in a concentration range of 0–3×10-3 mol/L. The mechanism with corresponding signal inhibition values for urea could be seen in Figs. 3b and d. Similarly, creatinine (Cre) was detected based on its capability to form a complex with copper ions. The Cre-Cu2+ ion could easily be adsorbed onto the surface of MXene, which during SWV sweep exhibited strong signals related to the redox behavior of copper (Fig. 3c). The current response obtained from the stripping peaks of Cu2+ ion was found linear with the concentration of Cre present in the system. This method enabled detection of Cre in a concentration range of 10–400 μmol/L with a LOD value of 1.2 μmol/L. The constructed microfluidic devise also demonstrated excellent working potential when tested for the renal function analysis of 105 healthy people compared to the conventional biochemical assays. Similarly, the ratiometric approach has also been considered for detecting piroxicam drug [48]. Here, Ti3C2Tx was modified with Cu NPs, to produce a stable hybrid platform capable of anchoring MB dye to act as a ratiometric probe. The hybrid system detected piroxicam both in tablet and human serum samples with acceptable recoveries. The working range, in this case, was described between 0.1 μmol/L and 80 μmol/L with a LOD value of 0.05 μmol/L. In another approach, Lei et al. [49] constructed a wearable sensor for invasive detection of glucose and lactase using the enzyme immobilization approach. The invasive sensors usually suffer from poor signal response, low-shell life and compromised sensitivity. Here, the use of MXene coupled with unique electrode configuration, allowed sufficient O2 supply throughout the measurement, providing high reliability during intensive measurement. In addition, the sensor utilized a ratiometric approach, which subsequently minimized signal fluctuation. This sensor was capable of detecting glucose in a range of 1.0 μmol/L to 0.015 mol/L, with a LOD value of 0.33 μmol/L. The lactate concentration could be detected in a range of 1.0 μmol/L to 0.022 mol/L with a LOD of 0.67 μmol/L. Moreover, the wrist band sensor could also be employed for pH detection. Table 1 comprises the electrochemical and biosensor reports utilizing MXene directly or indirectly for detecting various chemical and biological species. From the perspective of electrochemical (bio) sensors, MXenes' have shown rapid growth. However, there are still immense possibilities to utilize and configure MXenes to produce rapid yet sensitive and reliable signal output.
|
Download:
|
| Fig. 3. (a–c) The detection mechanism for UA, urea, and creatinine using MXene-based microfluidic device, (d–f) the corresponding signal generation in reference to MB signal taken as a standard for ratiometric quantification. Reprint with permission [47]. Copyright 2019, John Wiley and Sons. | |
|
|
Table 1 MXene-based electrochemical and biosensors. |
4. Application of MXene in gas sensors
Gas detection is a proportionally growing research area based on its widening scope in air quality control, pollution monitoring, therapeutics, diagnosis, and breath analysis. Apart from reactive gases such as H2S and NO2, the detection of volatile organic compounds (VOCs) at a concentration lower than parts per million (ppm) level, is highly crucial for early diagnosis of severe illnesses, including peptic ulcers. The two crucial challenges to devise a sensitive gas sensor include low electrical noise and high signal throughput [50]. This could only be achieved when the detecting platform supports high conductivity simultaneously with abundant active sites for interfacial interactions. In the case of conventional materials such as metal oxides, graphene, and phosphorous black (PB), a trade-off relation exists recognizing them as suitable yet not the favorable materials for gas sensing applications [51-56]. MXenes in the case of gas sensing, offers ample advantages, including a robust conductive surface with adequate surface dangling functionalities and active sites for gas adsorption. S. J. Kim et al. [57] extensively studied MXene (Ti3C2Tx) as substrate to detect gases such as acetone (CH3COCH3), ethanol (C2H5OH), ammonia (NH3), propanol (C2H5CHO) and reactive gases like nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon dioxide (CO2). The response of gas was evaluated against relative change in the resistance of Ti3C2Tx substrate post adsorption of gas molecules normalized against the baseline resistance of N2 gas. Unlike the conventional materials, Ti3C2Tx demonstrated positive resistance change for all the gases regardless of their nature. This implies the universal gas adsorption capability of MXene. Apparently, the gas molecules can interact with the surface-bound functionalities of Ti3C2Tx, which reduces its conductivity and thereby changes the resistance, i.e., signal response. In addition, the maximal response of each gas was determined to be 0.97%, 1.7%, 0.8% and 0.88% for acetone, ethanol, ammonia, and propanal, with a relatively lower response for NO2, SO2 and CO2 gases. This response was further examined to vary with the thickness of the MXene sheets, whereas the DFT theory, further confirmed that the metal–like conductivity and strong adsorption energy of the surface-bound moieties were responsible for the sensitive response of the sensor. This was interesting as it presumed that the specific functionalization of Ti3C2Tx could be considered to achieve selective gas sensing.
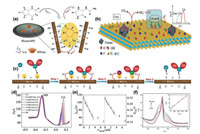
To achieve greater signal sensitivity, particularly for VOC gases (i.e., acetone, methanol and ethanol), W. Yuan et al. [58] transformed MXene into a 3D polymeric network. This 3D network was obtained by an electro-spinning approach where an aqueous solution of positively charged polymer PVA/PEI was mixed with negatively charged Ti3C2Tx. The electrostatic interaction later enabled self-assembly of MXene flakes over polymer, where electro-spinning allowed the formation of the 3D porous fibrous network. This network-based on its inter-connected porous nature allowed large diffusion of gas molecules, which enabled detection of VOC gases even at a low concentration range of 0.1 ppm to 0.17 ppm with detection signal measurable up to 50 ppb. The sensing capability of this 3D porous network was also compared with the pristine MXene, where the exposure to 5 ppm of acetone, methanol, and ethanol generated signal response twice that of pristine Ti3C2Tx, implying the importance of 3D networks in achieving a boosted signal response. In a much recent approach, E. Lee et al. [59] experimented on vanadium-based MXene material (V2CTx) for developing a gas sensor. Although much of the early work related to the gas sensor is based on Ti3C2Tx, this study opens up new routes of utilizing MXenes with different compositions as promising gas-sensing materials. The delaminated V2CTx sheets (Fig. 4) were utilized to construct an inter-digitated platinum electrode, which was then used to detect polar and non-polar gases including hydrogen and methane. The responses for ethanol, acetone, ammonia, methane, hydrogen and hydrogen sulfide were measured to be 0.0816, 0.0226, 0.0166, 0.0167, 0.2435 and 0.005, respectively (Fig. 4c). Unlike the Ti3C2Tx, which is more sensitive to ammonia [60], the vanadium-based MXene had a relatively greater sensitivity towards hydrogen gas. This implies that the change in the composition of MXenes could result in different interfacial interactions, shifting the selectivity of the devised platform towards different gases. In another approach, the interlayer swelling of Ti3C2Tx was carried out to understand its effect on gas sensing capabilities.
|
Download:
|
| Fig. 4. (a, b) The delamination and electrode fabrication process for vanadium-based MXene, i.e., V2CTx. (c) Typical response for each gas under fixed concentrations (100 ppm) with corresponding response times, and resistance v/s time measurement curves. Reprint with permission [59]. Copyright 2019, American Chemical Society. | |
For the conventional 2D materials, i.e., graphene, MoS2 sheets, the surface adsorption is a dominant phenomenon which can affect the channel conductivity when exposed to gases [55]. In the case of MXene, since interlayer swelling could drastically change the surface properties, the gas detection might rely on mechanism not only related to adsorption. E. Lee et al. [59] studied the in-situ change of interlayer distance upon the inclusion of responsive and non-responsive gases to Ti3C2Tx channels. Interestingly, the Ti3C2Tx films were found to selectively swell in the presence of ethanol vapors whereas no swelling was observed in the case of CO2 gas. In addition, the use of sodium ion for intercalation had a positive effect on the degree of swelling upon the addition of ethanol vapors. This is particularly interesting, as it can extend the understanding of gas sensing mechanism over Ti3C2Tx sheets, as previously it was thought that the surface interaction of gas molecules with Ti3C2Tx functionalities was dominant factor reasonable for changing the conductivity, whereas now this pre-intercalation could be used to define a selective and optimum pathway to devise reliable gas sensing platform. At present, the utilization of MXenes in gas detection sensors is limited. There is a dire need to understand the mechanisms, particularly to distinguish whether or not the response signal is adsorption based or related to the surface functionalities of MXenes.
5. MXenes in electro-chemiluminescence and fluorescent sensorsAmong the most recently evolving area, where the use of MXene is still at the primary stage, includes the development of electrochemiluminescence (ECL) & fluorescent probes for sensing and biosensing applications. For the common ECL platform which usually comprises of chemical reagents such as luminol, metal cluster compounds, or most often ([Ru(bpy)3]2+) to produce ECL signal upon interaction with the target analyte. The interaction could either enhance or discourage the chemiluminescence of the material, thereby enabling the quantification of target species. The conventional approach requires immobilization of [Ru(bpy)3]2+ onto the electrode surface using π-π stacking, electrostatic interaction, or physical embedding. This electrostatic interaction, which is most common and easy to adopt, requires not only a high surface area for greater adsorption of the probing agent but also an adequate quantity of negatively charged functionalities to enable firm interaction. Moreover, high electronic conductivity is still necessary to produce a measurable ECL signal readout.
The conventional materials, such as graphene, or its composites with metal and metal oxide [61-64], despite proven reliability, have compromised conductivity when integrated within the composite material. This, coupled with low aqueous dispersibility, and complex protocols, have encouraged scientist to explore new materials with superior properties. MXenes, particularly with their surface configuration and negative functionalities, i.e., —OH and —F, could be a potential material for ELC sensors. The high negative charge-density coupled with the large surface area could allow adequate immobilization of ECL probing agent, i.e., ([Ru(bpy)3]2+, whereas the robust conductivity of MXenes can also facilitate charge-transportation, which would encourage ECL signal enhancement. In view of such advantages, Y. Fang et al. [65] designed an ECL sensor for label-free singlenucleotide mismatch discrimination. Among most mutations, the single nucleotide mismatch is widely common. Thus, discrimination of such mismatch could be helpful in early disease diagnosis Since guanine and adenine bases in DNA can interact and enhance ECL signals of [Ru(bpy)3]2+ probe, using Ti3C2Tx as a highly conductive platform to immobilize the [Ru(bpy)3]2+ would allow easy discrimination of single nucleotide mismatch. Fig. 5 shows the generalized schematic configuration of this platform. Here, TPA was used as a representative co-reactant, which resulted in 32 folds high ECL signal intensity for [Ru(bpy)3]2+. The system demonstrated excellent feasibility when tested for single-nucleotide mismatch using oligonucleotides containing p53 gene segment as a model target. In the presence of 100 nmol/L P-MA with CA mismatch ECL intensity raised to the factor of 3.5 compared to P-CC at the same concentration. The ECL sensor was capable of distinguishing the mismatch even at a low concentration of 1.0 nmol/L. In a similar approach, H. Zhang et al. [66] utilized Ti3C2Tx nanosheets as carriers to immobilize aptamer for the detection of exosomes using the ECL approach. The large surface area and excellent conductivity enabled greater immobilization of aptamer molecules, which upon interaction with exosomes, enhanced the ECL signal of the luminol probing agent. The described strategy enabled sensitive detection of MCF-7 exosomes without the need of any co-rectors such as H2O2, whereas detection sensitivity of 125 particles/μL is achievable, which is 100 times lower than the commonly used ELISA technique. In regard to the fluorescent probes, MXenes, particularly Ti3C2Tx derived quantum dots, have been synthesized and explored in the area of intracellular pH detection [67]. The variation of intracellular pH is a crucial factor that could easily identify abnormal growth or cell function in case of diseases like cancer or Alzheimer's. The conventional fluorescent probes, which are basically organic molecules and semiconductor quantum dots despite their promising potential, are unfavorable candidates due to poor photo-stability, cytotoxicity, poor biocompatibility, and solubility. In this context, X. Chen et al. [67] fabricated polyethyleneimine (PEI) passivated Ti3C2 QDs using sonication and hydrothermal approach. The functionalized Ti3C2 QDs exhibited well-defined excitation wavelength and pH-dependent blue photoluminescence characteristics. These inherent characteristics allowed high quantum yield exhibition (7.13%), which then enabled the production of a ratiometric photoluminescence probe for monitoring intercellular pH variation. The ratiometric signal measurement was achieved by combining Ti3C2 QDs with pH insensitive [Ru(dpp)3]Cl2, probe. This enabled a stable signal response with minimum signal fluctuation. In a similar manner, X. Peng et al. [68] used the fluorescence detection approach to construct Human papillomavirus (HPV), sensitive platform based on ultra-thin Ti3C2Tx NSs. Here, the ultra-thin Ti3C2Tx NSs were used to support, dye-labeled ssDNA as a fluorescent probe. The fluorescent intensity of the dye is quenched post adsorption, which later could be recovered after its interaction with the complementary dsDNA. This mechanism allowed the detection of HPV-18 genes with signal sensitivity measurable up to 100 pmol/L. The platform was also evaluated to detect PCR amplified HPV-18 obtained from cervical scrapes samples.
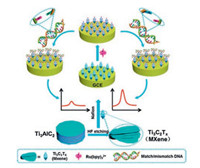
|
Download:
|
| Fig. 5. Illustration representing biosensor configuration over glassy carbon electrode immobilized with DNA using Ti3C2Tx sheets for single-nucleotide mismatch discrimination. Reprint with permission [65]. Copyright 2019, Elsevier Publisher. | |
6. Conclusions and future prospective
MXenes, which have emerged as unique 2D materials with graphene-like characteristics, have already made their impact in many rapidly expanding research areas. The promising surface chemistry, ease of functionalization, high hydrophilicity, and excellent conductivity with rapid yet easy synthesis protocol have recognized MXenes as a promising alternative for graphene. Though graphene is a well-studied 2D material in many areas, MXenes have only recently started to emerge as superior material in fields such as batteries, supercapacitors, and more recently electrochemical sensors. The area of electrochemical sensors, including biosensor, electro-chemiluminescence, and fluorescent is relatively new and the use of MXenes here as a transducer, immobilization platform, and as an active substrate is slowly gaining attention. The Ti3C2Tx-MXene form, based on its favorable surface chemistry, high surface area, redox activity, and optical characteristics, is being used to construct a sensitive sensor for pesticides, biomolecules, biomarkers, and blood component analysis. At present, the growing utilization of MXenes is within the area of electrochemical sensors or biosensors owing to high conductivity and stiff surface morphology that can easily support substantial immobilization and produce high signal throughput. The fast electron transportation with inherent negative surface functionalization (i.e., OH, —O and —F groups) can generate an intense electrochemical signal, capable of achieving lower detection limits and broader detection ranges. This clearly anticipates MXenes' growing fame and its future potential to drive the biosensor from the lab to the commercial market. Though MXenes and their derivatives are attracting a lot of audiences, there are still some obstacles that need to be trounced before realizing the true potential of this material in sensory devices. Among them, the selectivity of surface termination on MXenes is still challenging. When it comes to biosensors, fluorine termination is not favorable in certain conditions; thus, a suitable procedure for the removal of such functional groups could be beneficial for designing biosensor for specific biomedical applications. In addition, the oxidative stability of MXenes in aerated solution is another challenge to overcome before designing practical biosensors. Although efforts have been made to increase the stability of MXene-films by controlling the morphology and forming hybrids, the oxidative stability of MXenes still remains a big challenge to achieve high signal reproducibility, when it comes to electrochemical or bioelectrochemical sensors. This is especially a problem when designing a high-throughput biosensor utilizing a few-layer thick Ti3C2Tx. Since this oxidation usually results in surface-bound TiO2 particles [69], the partially oxidized MXenes could presumably be a potential candidate for a more advanced photo-electrochemical (PEC) sensor systems. The use of MXenes in PEC devises as pristine or hybrid form could drastically enhance signal sensitivity owing to the photoactive TiO2, which apparently has an adverse effect on the stability of MXenes. This would not only open up a new area of research but also take a step forward in understanding and utilization of MXenes in point of care devices. Moreover, from the synthesis perspective, out of 1/8 MXene phases, at present Ti3C2Tx exfoliated MXene is currently under investigation for sensory devices. The application of other phases in the electrochemical sensor systems is still an un-explored area with the benefit of obtaining superior 2D material.
The use of MXene in gas sensors has shown tremendous potential both in terms of selectivity and sensitivity. Unlike conventional materials, MXene-based surfaces are highly responsive to both volatile and non-volatile gases. However, the complete understanding of MXenes' working mechanism, particularly the interactions that drive the selectivity of MXene based substrates towards certain gases, is still unclear. In general, the surface interactions of gas molecules with the dangling moieties of MXene are the responsible forces that drive this selectivity. However, the new methods involving pre-intercalation and swelling, besides the composition of MXenes (i.e., Ti3C2Tx or V2CTx) have proven to influence the selectivity of the sensor under construction [57, 59]. Thus, an in-depth interpretation that could define the signal mechanism as adsorptive or categorical is a subject of attention. This would not only contribute to achieving superior gas responses but also open up pathways leading to the practical use of MXenebased resistive sensors in therapeutics and clinical applications.
In this review, most of the methods, procedures, and detection approaches clearly anticipate MXenes' superiority in achieving high signal sensitivity, excellent selectivity, and most important of all the capability to be integrated into devices for practical application. However, there are still many grey areas where the use of MXenes requires a better understanding with many challenges yet to be met by the sensor community before this new family of 2D materials could completely replace graphene as a better equivalent.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (NSFC, Nos. 51572011 and 51802012).
| [1] |
P. Zhang, Q. Zhu, Z. Guan, ChemSusChem 13 (2020) 1621-1628. DOI:10.1002/cssc.201901497 |
| [2] |
J. Lu, A. Thore, R. Meshkian, et al., Cryst. Growth Des. 17 (2017) 5704-5711. DOI:10.1021/acs.cgd.7b00642 |
| [3] |
J. Zhou, X. Zha, X. Zhou, et al., ACS Nano 11 (2017) 3841-3850. DOI:10.1021/acsnano.7b00030 |
| [4] |
P. Zhang, D. Wang, Q. Zhu, et al., Nano-Micro Lett. 11 (2019) 81. DOI:10.1007/s40820-019-0312-y |
| [5] |
H. Liu, X. Zhang, Y. Zhu, et al., Nano-Micro Lett. 11 (2019) 65. DOI:10.1007/s40820-019-0296-7 |
| [6] |
Y.T. Liu, P. Zhang, N. Sun, et al., Adv. Mater. 30 (2018) 1707334. DOI:10.1002/adma.201707334 |
| [7] |
Q. Zhao, Q. Zhu, J. Miao, et al., Small (2019) 1904293.
|
| [8] |
G.K. Nasrallah, M. Al-Asmakh, K. Rasool, K.A. Mahmoud, Environ. Sci. Nano 5 (2018) 1002-1011. DOI:10.1039/C7EN01239J |
| [9] |
L. Yu, L. Hu, B. Anasori, et al., ACS Energy Lett. 3 (2018) 1597-1603. DOI:10.1021/acsenergylett.8b00718 |
| [10] |
J. Zhu, E. Ha, G. Zhao, et al., Coord. Chem. Rev. 352 (2017) 306-327. DOI:10.1016/j.ccr.2017.09.012 |
| [11] |
A. Sinha, H. Zhao, Y. Huang, et al., Trends Analyt. Chem. 105 (2018) 424-435. DOI:10.1016/j.trac.2018.05.021 |
| [12] |
R.A. Soomro, Z.H. Ibupoto, S.T.H. Sherazi, et al., Mater. Express 5 (2015) 437-444. DOI:10.1166/mex.2015.1252 |
| [13] |
Z. Geng, X. Kong, Q. Li, J. Ke, J. Zeng, Curr. Opin. ElectroChem. 17 (2019) 7-15. DOI:10.1016/j.coelec.2019.04.008 |
| [14] |
E.Saab, M.Asteazaran, G.Cespedes, A.M.C.Luna, J.Electrochem.Soc.(2019)1559.
|
| [15] |
Z. Xia, S. Guo, Chem. Soc. Rev. 48 (2019) 3265-3278. DOI:10.1039/C8CS00846A |
| [16] |
L. Li, J. Liang, L. Qin, D. Chen, Y. Huang, J. Mater. Chem. C 7 (2019) 6872-6878. DOI:10.1039/C9TC02147G |
| [17] |
A. Shahzad, K. Rasool, M. Nawaz, et al., Chem. Eng. J. 349 (2018) 748-755. DOI:10.1016/j.cej.2018.05.148 |
| [18] |
Y. Sun, X. Meng, Y. Gao, et al., Catal. Sci. Technol. 9 (2019) 310-315. DOI:10.1039/C8CY02240B |
| [19] |
C.J. Zhang, S. Pinilla, N. McEvoy, et al., Chem. Mater. 29 (2017) 4848-4856. DOI:10.1021/acs.chemmater.7b00745 |
| [20] |
Z. Zhang, H. Li, G. Zou, et al., ACS Sustain. Chem. Eng. 4 (2016) 6763-6771. DOI:10.1021/acssuschemeng.6b01698 |
| [21] |
M. Naguib, M. Kurtoglu, V. Presser, et al., Adv. Mater. 23 (2011) 4248-4253. DOI:10.1002/adma.201102306 |
| [22] |
P.K. Kalambate, N.S. Gadhari, X. Li, et al., Trends Analyt. Chem. (2019) 115643.
|
| [23] |
S. Zhang, H. Liu, B. Cao, et al., J. Mater. Chem. A 7 (2019) 21766-21773. DOI:10.1039/C9TA07357D |
| [24] |
N. Sun, Q. Zhu, B. Anasori, et al., Adv. Funct. Mater. 29 (2019) 201906282. |
| [25] |
Q. Zhao, Q. Zhu, J. Miao, P. Zhang, B. Xu, Nanoscale 11 (2019) 8442-8448. DOI:10.1039/C8NR09653H |
| [26] |
J. Ran, G. Gao, F.T. Li, et al., Nat. Commun. 8 (2017) 13907. DOI:10.1038/ncomms13907 |
| [27] |
Z. Ling, C.E. Ren, M.Q. Zhao, et al., Proc. Natl. Acad. Sci. 111 (2014) 16676-16681. DOI:10.1073/pnas.1414215111 |
| [28] |
H. Liao, X. Guo, P. Wan, G. Yu, Adv. Funct. Mater. 29 (2019) 1904507. DOI:10.1002/adfm.201904507 |
| [29] |
D. Wu, M. Wu, J. Yang, et al., Mater. Lett. 236 (2019) 412-415. DOI:10.1016/j.matlet.2018.10.150 |
| [30] |
P.A. Rasheed, R.P. Pandey, K.A. Jabbar, J. Ponraj, K.A. Mahmoud, Anal. Methods 11 (2019) 3851-3856. DOI:10.1039/C9AY00912D |
| [31] |
R. Huang, S. Chen, J. Yu, X. Jiang, Ecotoxicol. Environ. Saf. 184 (2019) 109619. DOI:10.1016/j.ecoenv.2019.109619 |
| [32] |
Y. Zhang, X. Jiang, J. Zhang, H. Zhang, Y. Li, Biosens.Bioelectron. 130 (2019) 315-321. DOI:10.1016/j.bios.2019.01.043 |
| [33] |
P.A. Rasheed, R.P. Pandey, K. Rasool, K.A. Mahmoud, Sens. Actuator. B-Chem. 265 (2018) 652-659. DOI:10.1016/j.snb.2018.03.103 |
| [34] |
F. Shahzad, A. Iqbal, S.A. Zaidi, S.W. Hwang, C.M. Koo, J. Ind. Eng. Chem. 79 (2019) 338-344. DOI:10.1016/j.jiec.2019.03.061 |
| [35] |
J. Zheng, B. Wang, A. Ding, B. Weng, J. Chen, J. Electroanal. Chem. 816 (2018) 189-194. DOI:10.1016/j.jelechem.2018.03.056 |
| [36] |
L. Lorencova, T. Bertok, J. Filip, et al., Sens.Actuator. B-Chem. 263 (2018) 360-368. DOI:10.1016/j.snb.2018.02.124 |
| [37] |
L. Lorencova, T. Bertok, E. Dosekova, et al., Electrochim.Acta 235 (2017) 471-479. DOI:10.1016/j.electacta.2017.03.073 |
| [38] |
D. Song, X. Jiang, Y. Li, et al., J. Hazard. Mater. 373 (2019) 367-376. DOI:10.1016/j.jhazmat.2019.03.083 |
| [39] |
Y. Jiang, X. Zhang, L. Pei, et al., Chem. Eng. J. 339 (2018) 547-556. DOI:10.1016/j.cej.2018.01.111 |
| [40] |
J. Zheng, J. Diao, Y. Jin, et al., J. Electrochem. Soc. 165 (2018) 227-231. DOI:10.1149/2.0051807jes |
| [41] |
F. Wang, C. Yang, C. Duan, et al., J. Electrochem. Soc. 162 (2015) 16-21. DOI:10.1149/2.0371501jes |
| [42] |
J. Zheng, B. Wang, Y. Jin, et al., Microchim. Acta 186 (2019) 95. DOI:10.1007/s00604-018-3220-9 |
| [43] |
R.B. Rakhi, P. Nayak, C. Xia, H.N. Alshareef, Sci. Rep. 6 (2016) 36422. DOI:10.1038/srep36422 |
| [44] |
L. Wu, X. Lu, Dhanjai, et al., Biosens. Bioelectron. 107 (2018) 69-75. DOI:10.1016/j.bios.2018.02.021 |
| [45] |
S. Kumar, Y. Lei, N.H. Alshareef, M.A. Quevedo-Lopez, K.N. Salama, Biosens. Bioelectron. 121 (2018) 243-249. DOI:10.1016/j.bios.2018.08.076 |
| [46] |
S. Zhou, C. Gu, Z. Li, et al., Appl. Surf. Sci. (2019) 143889.
|
| [47] |
J. Liu, X. Jiang, R. Zhang, et al., Adv. Funct. Mater. 29 (2019) 1807326. DOI:10.1002/adfm.201807326 |
| [48] |
R. Zhang, J. Liu, Y. Li, ACS Sens. 4 (2019) 2058-2064. DOI:10.1021/acssensors.9b00654 |
| [49] |
Y. Lei, W. Zhao, Y. Zhang, et al., Small 15 (2019) 1901190. DOI:10.1002/smll.201901190 |
| [50] |
H.G. Shiraz, Int. J. Hydrog. Energy 42 (2017) 15966-15972. DOI:10.1016/j.ijhydene.2017.05.045 |
| [51] |
D. Kwak, Y. Lei, R. Maric, Talanta 204 (2019) 713-730. DOI:10.1016/j.talanta.2019.06.034 |
| [52] |
Y. Sun, M. Ma, B. Tang, et al., J. Alloys. Compd. 808 (2019) 151721. DOI:10.1016/j.jallcom.2019.151721 |
| [53] |
Y.C. Wang, Z.S. Sun, S.Z. Wang, et al., J. Mater. Sci. 54 (2019) 14055-14063. DOI:10.1007/s10853-019-03877-y |
| [54] |
Y. Zhou, Y. Wang, Y. Guo, Mater. Lett. 254 (2019) 336-339. DOI:10.1016/j.matlet.2019.07.119 |
| [55] |
M. Donarelli, L. Ottaviano, Sensors 18 (2018) 3638. DOI:10.3390/s18113638 |
| [56] |
T. Srivastava, R. Jha, IEEE Photonics Technol. Lett. 30 (2018) 319-322. DOI:10.1109/LPT.2017.2787057 |
| [57] |
S.J. Kim, H.- J. Koh, C.E. Ren, et al., ACS Nano 12 (2018) 986-993. DOI:10.1021/acsnano.7b07460 |
| [58] |
W. Yuan, K. Yang, H. Peng, F. Li, F. Yin, J. Mater. Chem. A 6 (2018) 18116-18124. DOI:10.1039/C8TA06928J |
| [59] |
E. Lee, A.V. Mohammadi, Y.S. Yoon, M. Beidaghi, D.J. Kim, ACS Sens. 4 (2019) 1603-1611. DOI:10.1021/acssensors.9b00303 |
| [60] |
B. Xiao, Y.C. Li, X.F. Yu, J.B. Cheng, Sens. Actuator. B-Chem. 235 (2016) 103-109. DOI:10.1016/j.snb.2016.05.062 |
| [61] |
Y. Xia, M. Liu, L. Wang, et al., Biosens. Bioelectron. 92 (2017) 8-15. DOI:10.1016/j.bios.2017.01.063 |
| [62] |
Y. He, J. Li, Y. Liu, Anal. Chem. 87 (2015) 9777-9785. DOI:10.1021/acs.analchem.5b02048 |
| [63] |
M. Li, C. Wang, L. Chen, D. Liu, Anal. Chim. Acta 1090 (2019) 57-63. DOI:10.1016/j.aca.2019.09.018 |
| [64] |
X.L. Zhang, X. Li, X.T. Li, et al., Sens. Actuator. B-Chem. 282 (2019) 927-935. DOI:10.1016/j.snb.2018.11.113 |
| [65] |
Y. Fang, X. Yang, T. Chen, et al., Sens. Actuator. B-Chem. 263 (2018) 400-407. DOI:10.1016/j.snb.2018.02.102 |
| [66] |
H. Zhang, Z. Wang, Q. Zhang, F. Wang, Y. Liu, Biosens. Bioelectron. 124-125 (2019) 184-190. DOI:10.1016/j.bios.2018.10.016 |
| [67] |
X. Chen, X. Sun, W. Xu, et al., Nanoscale 10 (2018) 1111-1118. DOI:10.1039/C7NR06958H |
| [68] |
X. Peng, Y. Zhang, D. Lu, Y. Guo, S. Guo, Sens. Actuator. B-Chem. 286 (2019) 222-229. DOI:10.1016/j.snb.2019.01.158 |
| [69] |
Z. Guan, X. Wang, T. Li, et al., J. Mater. Sci. Technol. 35 (2019) 1977-1981. |
 2020, Vol. 31
2020, Vol. 31