b South China Advanced Institute for Soft Matter Science and Technology, South China University of Technology, Guangzhou 510640, China
Stimuli-responsive gels are intriguing materials for various applications ranging from flexible electronics, artificial muscles, actuators, and sensors to the mimicry of extracellular matrix for cell culture and tissue regeneration [1, 2]. These applications usually require the gels to exhibit responsiveness to multiple triggers such as acid/base, redox signals, enzymes, heat, and light [3-5]. The early generation of responsive gels is designed based on the inherent property of used polymers, i.e., temperature-sensitive poly(N-isopropyl acrylamide) and pH-sensitive poly(acrylic acid). To expand the stimuli-responsiveness of the gels, dynamic covalent linkages such as boronate esters, disulfide bonds, thioesters, hemiaminal, and imine bonds or reversible non-covalent host-guest pairs such as cyclodextrin/azobenzene were introduced to form polymer networks [6-11]. These gels could respond to various stimuli by specific material design, but usually require the pre-synthesis of macromolecular building blocks with chemical reactive ligands on the backbone or side chains. This involves high complexity in material synthesis, which limits the large scale and repeatable preparation of corresponding gels [12]. An alternative strategy is synthesis of low molecular weight gelators containing dynamic covalent connections that can form fibrous networks via supramolecular assembly [13-17]. These supramolecular gels can be facilely prepared, but usually only respond to a single or two stimuli. In addition, the non-covalent forces holding the supramolecular gel are relatively weak, and this may lead to low gel stability [18]. It is hypothesized that dynamic covalent linkages can be used to directly crosslink small molecules with multiple functional groups [19, 20], yielding a class of all-small-molecule dynamic covalent gels. Here, we report the preparation of such gels by direct gelation of natural occurring polyphenol tannic acid (TA) with a series of commercially available inorganic borate (1–4) or organic boronate (5–6) building blocks via reversible boronate ester bonds. Though the boronate esters with catechol, 1, 2 or 1, 3 diols were widely used as reversible linkages in responsive polymeric gels [21-26], the direct gelation of naturally occurring small molecules using this dynamic covalent chemistry is yet to be investigated. The formed gels are responsive to acid/base, oxidants or reducing chemicals depending on the chosen borate/boronate building blocks.
TA is a natural occurring hydrophilic polyphenol with a high density of pyrogallic acid or catechol groups on its structure. Its excellent aqueous solubility and multiple reactive catechol groups make it an ideal candidate in the preparation of all-small-molecule dynamic covalent hydrogels [27-30]. Here, commercially available inorganic borates including NaBO2, Na2B4O7, Na2BO3 and H3BO3/ NaOH were firstly used to crosslink the TA molecules in aqueous solution via boronate ester bonds (Fig. 1a). Take NaBO2 for example, the inorganic borate could form hydrogels with TA within a wide concentration ranges for both NaBO2 and TA (Fig. 1b). A yellow-colored and transparent hydrogel (Gel 1) was formed within minutes by gently mixing the two solutions together at ambient temperature. Rheology result in Fig. 1c showed that the storage modulus (G') of a representative gel is higher than the loss modulus (G") after 1200 s, which is usually considered as the gelation time. We then investigated the effects of NaBO2 and TA concentrations on the gel stiffness and gelation time (Figs. S1a-d in Supporting information). When the NaBO2 concentration increased from 0.3 mol/L to 0.4 mol/L, the storage modulus of hydrogel increased and the gelation time decreased. With a further increase in NaBO2 concentration, a reverse trend was observed. Assuming there are five binding sites on each TA molecule, 0.4 mol/L NaBO2 is theoretically to interact with 0.16 mol/L TA via the formation of catechol-borate diesters. Therefore, a maximum storage modulus and shortest gelation time were achieved at 0.4 mol/L NaBO2. More borates in the system will lead to increased catechol-borate monoesters, and decreased crosslinking degree in the gel. On the other hand, when the TA concentration was increased from 0.13 mol/L to 0.22 mol/L, the storage modulus increased, while the gelation time decreased. This is due to increased content of catechol-borate diesters and hydrogen bonding interactions in the network at higher TA concentrations. Like most hydrogels, the yielding TA/NaBO2 gel displayed shear-thinning (Fig. 1d), thixo-tropic (Fig. 1e), self-healing (Fig. 1f) properties, and could be processed to different shapes using specific molds (Fig. 1g).

|
Download:
|
| Fig. 1. TA/borate hydrogels. (a) Formation of TA/borate hydrogels by dynamic covalent connections between TA and several inorganic borates (Gel 1, NaBO2; Gel 2, Na2B4O7; Gel 3, NaBO3; Gel 4, H3BO3 and NaOH).(b) Phase diagram of TA/NaBO2 mixtures. Time-dependent rheology (c), shear-thinning (d) and thixotropic (e) properties of the TA/ NaBO2 hydrogel (Gel 1). (f) Self-healing behavior of Gel 1. (g) Gel 1 was fabricated into different shapes. | |
We next investigated the mechanism of TA/NaBO2 gelation in aqueous solution by solution 11B NMR spectroscopy. The samples were prepared at TA to NaBO2 molar ratios ranging from 0:1 to 0.26:1 in deuterated water at pH 5.5 (no gelation observed under this condition, pH 5.5 is the pH value for TA/NaBO2 mixture in Gel 1), and added into Teflon tubes for 11B NMR measurement. As shown in Fig. 2a, NaBO2 alone showed a sharp peak at 20.1 ppm at pH 5.5, which is the characteristic peak for tetrahedral borate anion [31]. After the addition of TA molecules, a new peak at 8.7 ppm was observed, and this peak is assigned to the borate monoester formed between catechol and tetrahedral borate anion. With increasing molar ratio of TA to NaBO2, the intensity for the peak at 8.7 ppm is increased, and another peak at 14.7 ppm assigned to the borate diesters is observed. The chemical shifts for catechol-borate monoesters and diesters are in accordance with the reference results [31, 32]. The catechol-borate diesters are the major driven force for gelation between TA and tetrahedral borate (Fig. 2b). Besides 11B NMR, we also characterized the TA/NaBO2 gel by a resonance Raman Spectroscopy. Compared to free TA or NaBO2, a new peak was observed at 1412 cm -1 which is assigned to catechol-borate esters in the formed gels (Fig. 2c). The responsive behaviors of Gel 1 to acid or base were then investigated. As shown in Fig. 2d and Fig. S2 (Supporting information), the formed TA/NaBO2 gel was relatively stable at pH 5.0–6.0, but rapidly dissolved in the presence of HCl or NaOH. This can be explained by the cleavage of catechol-borate diesters in acidic environments and hydrolysis/oxidation of TA in basic conditions [33-35]. These speculations were confirmed by 11B NMR results in Fig. 2d, where the catechol-borate diesters were disappeared in both cases.

|
Download:
|
| Fig. 2. Gelation and degradation mechanisms of the TA/borates hydrogels. (a) Solution 11B NMR spectra of TA/NaBO2 mixtures. The TA to NaBO2 molar ratios were ranged from 0:1 to 0.26:1 in D2O fixed at pH 5.5. (b) The formation of catechol-borate monoesters and diesters between TA and NaBO2. (c) Raman spectra of TA, NaBO2, and TA/NaBO2 hydrogels. (d) 11B NMR spectra of TA/NaBO2 in D2O at a molar ratio of 1:0.12 at different pH conditions. (e) The hydrolysis of Na2B4O7 in aqueous solution. (f) Time-dependent rheology of the TA/Na2B4O7 hydrogel (Gel 2). (g) 11B NMR spectra of NaBO2, Na2B4O7, H3BO3 in D2O. (h) TA/Na2B4O7 mixtures at various pH conditions. | |
According to the analysis above, tetrahedral borate is the key structure for TA/borate gelation. Therefore, other inorganic borates that could hydrolyze into tetrahedral borate have the potential of gelation with TA via the dynamic covalent chemistry. Na2B4O7 is a naturally occurring inorganic borate can be hydrolyzed to trigonal and tetrahedral borates at an appropriate molar ratio of 1:1 in aqueous solution (Fig. 2e). As shown in Fig. 2f, TA and Na2B4O7 also form a transparent and yellow-colored gel after gentle mixing (Gel 2). In the 11B NMR spectrum (Fig. 2g), a peak of 9.7 ppm is observed in the Na2B4O7 solution (0.4 mol/L, pH 9.3), this peak is the weighted average of trigonal-state (2.5 ppm) and tetrahedral-state (20.1 ppm) borates in solution, which exhibiting a fast exchange between the two states within the NMR time scale [31]. The content of tetrahedral borates in the Na2B4O7 solution can be modulated by facilely changing the solution pH, and this property can be used to tune the gel state. As shown in Fig. 2h, the TA/Na2B4O7 hydrogel is also pH-responsive and stable TA/Na2B4O7 gels were formed within the pH range of 5.0-6.0. Besides Na2B4O7, NaBO3 and a mixture of H3BO3 and NaOH at a molar ratio of 1:1 can be used to prepare dynamic covalent hydrogels with TA (Gel 3 and Gel 4, Fig. S3 in Supporting information).
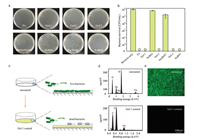
TA has been widely reported with antibacterial activity by destabilizing the bacteria membrane [36]. We further investigated the in vitro antibacterial activity of the prepared hydrogels. As shown in Figs. 3a and b, the natural polyphenol TA and all the TA/ borate hydrogels effectively inhibited the growth of E. coli, suggesting the potent antibacterial activity of TA and TA-containing gels. The borates NaBO2 and Na2B4O7 showed limited effect on the inhibition of E. coli growth. Interestingly, NaBO3 effectively killed the bacteria at an equal borate concentration. This can be explained by the yielding of hydrogen peroxide during the hydrolysis of NaBO3 in aqueous solution. The TA/NaBO2 hydrogels could be easily coated on substrates such as culture plates and glass slides due to the catechol groups on the gel surface (Fig. 3c). The SEM-EDS analysis in Fig. 3d suggests the successful coating of Gel 1 on a glass slide. After gel coating, the substrate effectively killed E. coli expressing green fluorescence protein (GFP, Fig. 3e). These results together suggest that the TA/ borate gels have potent antibacterial activity.
|
Download:
|
| Fig. 3. (a) Images of LB agar inoculated with E. coli suspensions incubated on TA/borate hydrogel-coated plates (Gel 1-Gel 3). TA, NaBO2, Na2B4O7 and NaBO3 were tested as controls. The white dots on the plates are live bacteria colonies. (b) Antibacterial activities of the hydrogels and chemicals in (a). (c) Coating of hydrogels on the surface of substrates. (d) Characterization of Gel 1 coated glass slide by SEM-EDS. Uncoated glass slide was characterized as a control. (e) Fluorescence images of uncoated and Gel 1 coated culture plates. The culture plates were incubated with E. coli expressing GFP. | |
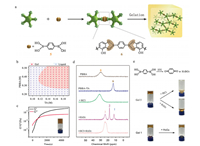
We further expanded the gel responsiveness by using organic boronates to crosslink with TA. 1, 4-Phenylenebisboronic acid (PBBA) with two boronate groups in the structure is expected to form catechol-boronate diesters for gelation (Fig. 4a) [21, 22]. TA and PPBA formed stable gels within a high concentration ranges for both chemicals (Fig. 4b), and the yielding gel (Gel 5) is yellow-colored with G' higher than G'' in the rheology studies (Fig. 4c). PBBA showed a broad peak at 31.5 ppm in 11B NMR, which is assigned to the trigonal-state of boronates on the chemical [37]. After the addition of TA, a new peak at 13.6 ppm is observed which is attributed to the formed catechol-boronate ester (Fig. 4d). The peak for catechol-boronate ester is disappeared in the presence of HCl, which is similar to the behavior of catechol-borate esters for Gel 1 and Gel 2 (Fig. 4e and Fig. S4 in Supporting information). Besides acid and base, the yielding gel is also responsive to oxidant chemicals such as H2O2, which is due to the oxidative degradation of PBBA into inorganic borates by H2O2 (Fig. 4e). This process is confirmed by the disappearance of the boronate ester peak in the 11B NMR upon H2O2 addition (Fig. 4d). In comparison, the gels prepared by TA and NaBO2 showed weak sensitivity to H2O2 under the same condition (Fig. 4e).
|
Download:
|
| Fig. 4. (a) Formation of TA/PBBA gel by dynamic covalent connections. (b) Phase diagram of TA/PBBA mixtures. (c) Time-dependent rheology of the TA/PBBA (Gel 5). (d) 11B NMR spectra of the TA/PBBA mixture in the presence of various triggers. (e) Acid- and H2O2-responsive behaviors of Gel 5. Gel 1 formed by TA and NaBO2 is relatively stable in the presence of H2O2. | |
Finally, a commercially available organic boronate 4-mercap-tophenylboronic acid (MPBA) was used as the boronate building block to prepare the dynamic covalent gels. MPBA was oxidized into dimers in the presence of DMSO and successfully formed a transparent gel with TA (Fig. 5a). 11B NMR spectra suggested the formation of catechol-boronate esters in the mixture (Fig. 5b). The formed gel (Gel 6) responsiveness to acid, base, H2O2 (Fig. 5c and Fig. S5 in Supporting information), which is similar to Gel 5 in Fig. 4. Interestingly, Gel 6 also degraded rapidly in the presence of DTT, which is distinct from Gel 5. The DTT responsiveness of Gel 6 is explained by the cleavage of disulfide in the gel structure into thiol groups, which destroyed the crosslinking networks in the gel.

|
Download:
|
| Fig. 5. (a) Concept of gelation between TA and MPBA. (b) 11B NMR spectra of MPBA and TA/MPBA mixture. (c) H2O2- and DTT-responsive behaviors of the TA/MPBA gel (Gel 6). Gel 5 consisted of TA and PBBA is relatively stable in the presence of DTT. | |
In summary, we reported a series of all-small-molecule dynamic covalent gels with antibacterial properties by direct gelation between TA and inorganic borates or organic boronates. In comparison with the responsive polymeric gels that require the pre-synthesis of macromolecular building blocks with chemical reactive ligands, the gels reported in this study are formed by direct gelation between natural occurring polyphenol TA and commercially available borate compounds. Besides facile construction, the all-small-molecule dynamic covalent hydrogels also show predictable degradation property. The hydrogels could be completely degraded into the small molecules upon multistimuli-responsiveness accord-ing to the chosen building blocks. The prepared dynamic covalent gels offer high flexibility to tune the physicochemical properties and responsiveness for various applications.
AcknowledgementsThis work is supported by the National Natural Science Foundation of China (No. 21725402) and the Shanghai Municipal Science and Technology Commission (No. 17XD1401600).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.013.
| [1] |
Y. Shi, Z. Wang, X. Zhang, et al., Chem. Commun. 51 (2015) 15265-15267. DOI:10.1039/C5CC05792B |
| [2] |
D. Wang, Y. Hu, P. Liu, D. Luo, Acc. Chem. Res. 50 (2017) 733-739. DOI:10.1021/acs.accounts.6b00581 |
| [3] |
W. Cao, X. Zhang, X. Miao, Z. Yang, H. Xu, Angew. Chem. Int. Ed. 52 (2013) 6233-6237. DOI:10.1002/anie.201300662 |
| [4] |
Z. Liu, T. Liu, Q. Lin, C. Bao, L. Zhu, Angew. Chem. Int. Ed. 54 (2015) 174-178. DOI:10.1002/anie.201409097 |
| [5] |
Z. Liu, Q. Lin, Y. Sun, et al., Adv. Mater. 26 (2014) 3912-3917. DOI:10.1002/adma.201306061 |
| [6] |
C.H. Fox, G.M. ter Hurrne, R.J. Wojtecki, et al., Nat. Commun. 6 (2015) 7417. DOI:10.1038/ncomms8417 |
| [7] |
J.M. Garcia, G.O. Jones, K. Virwani, et al., Science 344 (2014) 732-735. DOI:10.1126/science.1251484 |
| [8] |
K. Iwaso, Y. Takashima, A. Harada, Nat. Chem. 8 (2016) 625-632. DOI:10.1038/nchem.2513 |
| [9] |
Y. Takashima, S. Hatanaka, M. Otsubo, et al., Nat. Commun. 3 (2012) 1270. DOI:10.1038/ncomms2280 |
| [10] |
J. Lou, F. Liu, C.D. Lindsay, et al., Adv. Mater. 30 (2018) 1705215. DOI:10.1002/adma.201705215 |
| [11] |
R. Tamate, K. Takahashi, T. Ueki, A.M. Akimoto, R. Yoshida, Biomacromolecules 18 (2017) 281-287. DOI:10.1021/acs.biomac.6b01672 |
| [12] |
R. Guo, Q. Su, J. Zhang, et al., Biomacromolecules 18 (2017) 1356-1364. DOI:10.1021/acs.biomac.7b00089 |
| [13] |
J.M. Poolman, J. Boekhoven, A. Besselink, et al., Nat. Protoc. 9 (2014) 977-988. DOI:10.1038/nprot.2014.055 |
| [14] |
G.M. Peters, L.P. Skala, T.N. Plank, et al., J. Am. Chem. Soc. 136 (2014) 12596-12599. DOI:10.1021/ja507506c |
| [15] |
G.M. Peters, L.P. Skala, T.N. Plank, et al., J. Am. Chem. Soc. 137 (2015) 5819-5827. DOI:10.1021/jacs.5b02753 |
| [16] |
J. Zheng, R. Fan, H. Wu, et al., Nat. Commun. 10 (2019) 1604. DOI:10.1038/s41467-019-09601-3 |
| [17] |
J.Y. Zhang, L.H. Zeng, J. Feng, Chin. Chem. Lett. 28 (2017) 168-183. DOI:10.1016/j.cclet.2016.07.015 |
| [18] |
M. Singh, S. Kundu, M.A. Reddy, et al., Nanoscale 6 (2014) 12849-12855. DOI:10.1039/C4NR04064C |
| [19] |
J.H. Lee, J. Park, J.W. Park, et al., Nat. Commun. 6 (2015) 6650. DOI:10.1038/ncomms7650 |
| [20] |
H. Wang, Y. Cheng, Mater. Chem. Front. 3 (2019) 472-475. DOI:10.1039/C8QM00612A |
| [21] |
S. Lamping, T. Otremba, B.J. Ravoo, Angew. Chem. Int. Ed. 57 (2018) 2474-2478. DOI:10.1002/anie.201711529 |
| [22] |
A.R. Narkar, B. Barker, M. Clisch, J. Jiang, B.P. Lee, Chem. Mater. 28 (2016) 5432-5439. DOI:10.1021/acs.chemmater.6b01851 |
| [23] |
J.V. Accardo, J.A. Kalow, Chem. Sci. 9 (2018) 5987-5993. DOI:10.1039/C8SC02093K |
| [24] |
Y. Chen, W. Wang, D. Wu, M. Nagao, D.G. Hall, Biomacromolecules 19 (2018) 596-605. DOI:10.1021/acs.biomac.7b01679 |
| [25] |
T.T. Duncan, B.H. Berrie, R.G. Weiss, Appl. Mater. Interfaces 9 (2017) 28069-28078. DOI:10.1021/acsami.7b09473 |
| [26] |
M.E. Smithmyer, C.C. Deng, S.E. Cassel, et al., Macro. Lett. 7 (2018) 1105-1110. DOI:10.1021/acsmacrolett.8b00462 |
| [27] |
M.A. Rahim, M. Bjornmalm, T. Suma, et al., Angew. Chem. Int. Ed. 55 (2016) 13803-13807. DOI:10.1002/anie.201608413 |
| [28] |
J. Guo, B.L. Tardy, A.J. Christofferson, et al., Nat. Nanotechnol. 11 (2016) 1105-1111. DOI:10.1038/nnano.2016.172 |
| [29] |
J.H. Park, K. Kim, J. Lee, et al., Angew. Chem. Int. Ed. 53 (2014) 12420-12425. |
| [30] |
H. Fan, L. Wang, X. Feng, et al., Macromolecules 50 (2017) 666-676. DOI:10.1021/acs.macromol.6b02106 |
| [31] |
M. Bishop, N. Shahid, J. Yang, A.R. Barron, Dalton Trans. (2004) 2621-2634. |
| [32] |
G.M. Peters, J.T. Davis, Supramol. Chem. 26 (2014) 286-295. DOI:10.1080/10610278.2013.872782 |
| [33] |
Z. Liu, H. He, Acc. Chem. Res. 50 (2017) 2185-2193. DOI:10.1021/acs.accounts.7b00179 |
| [34] |
A. Gennari, C. Gujral, E. Hohn, et al., Bioconjug. Chem. 28 (2017) 1391-1402. DOI:10.1021/acs.bioconjchem.7b00080 |
| [35] |
J.W. Tomsho, S.J. Benkovic, J. Org. Chem. 77 (2012) 2098-2106. DOI:10.1021/jo202250d |
| [36] |
M. Daglia, Curr. Opin. Biotechnol. 23 (2012) 174-181. DOI:10.1016/j.copbio.2011.08.007 |
| [37] |
M.L. Stolowitz, C. Ahlem, K.A. Hughes, et al., Bioconjug. Chem. 12 (2001) 229-239. DOI:10.1021/bc0000942 |
 2020, Vol. 31
2020, Vol. 31 

