4-Nitrophenol (4-NP) is among the mostly used chemicals in the industry [1, 2]. However, the elimination of 4-NP from water waste is an important issue in environmental science because 4-NP is a general organic pollutant generated during industrial or agricultural processes. Various methods have been applied to eliminate 4-NP, mainly includes: adsorption, photocatalysis, advanced oxidation processes, catalytic chemical oxidation, and nitro group reduction [3-7]. Among these methods, the reduction has been commonly adopted for the obvious advantages of mild reaction conditions, simple reaction treatment, and environmental friendliness. Moreover, its reduction product (aminophenol) can be reused. Therefore, it is virtually significant to develop effective catalysts for the reduction of 4-NP.
Due to their unprecedented activities, noble metal nanoparticles have attracted significant interest in catalysis science and engineering. Palladium nanoparticles (Pd NPs) catalysts are now obtaining attention because of their unique catalytic activity in the treatment of wastewater containing 4-NP [8]. Moreover, shape-controlled palladium nanomaterials have important application value in the field of catalysis. Moreover, ligands play a key role in the preparation of nanomaterials with different morphologies [9]. Herein, Pd nanoflowers (Pd NFs) was firstly developed using amino-functionalized fullerene (C60-NH2) as a supporting platform by a facile self-assembly hydrothermal approach (referred to as Pd NFs/C60-NH2). Assembly growth prepared Pd NFs/C60-NH2 exhibited further improvement of catalytic activity and stability for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) by UV–vis spectra. We believe that fullerene nano-platforms supported novel metal nanoflowers will offer promising applications in catalysis.
Amine functionalized fullerene (C60-NH2) was synthesized according to the procedure of previous study [10]. The resulting dark brown solid products (C60-NH2) were dried in vacuum at 60 C for 24 h. Pd nanoflowers were synthesized as follows. Palladium chloride precursor (PdCl2≥99.9%) in the amount of 0.02 mmol was dissolved in two drops of HCl (36.5%), then added into a mixture of 40 mL N, N-dimethylformamide (DMF) of with 1 mg C60-NH2 (the mass rate of Pd: C60-NH2 is 1:2) together. The combination was then placed into a TeflonⓇ-lined autoclave. The autoclave was purged with N2 for three times. After that, heated and conditioned at 140 C for 6 h without stirring, followed by separating and washing with ethanol, vacuum drying at 60 C overnight. The yield of Pd NF/C60-NH2 was ca. 90%. The catalytic activity of different Pd nanocomposites was tested by the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) using NaBH4 as the reducing agent. In a typical test, 4-NP (2.3 mmol/L) was first mixed with fresh NaBH4 (0.032 mol/L) aqueous solution (the molar ratio of NaBH4 to 4-NP was 200:1) from which 1 mL of the mixture solution was transferred to the quartz cuvette. A 10 μL portion of catalyst Pd NFs/C60-NH2 (1 mg/mL) was introduced into the cuvette followed by rapid mixing with the pipetting over 2 s. The reaction kinetics at room temperature was monitored at different time by UV–vis scans between 200 nm and 500 nm. The maximum absorption was recorded at 400 nm and used for evaluating the concentration of 4-NP.
To increase the effective anchoring sites, ethylenediamine (EA) rich of amino-group (-NH2) is introduced to the surface of C60 by the intrinsic strong electron deficient olefins properties of C60. The Pd NFs/C60-NH2 nanoflowers were assembled successfully without stirring. The representative transmission electron microscopic (TEM) image of Pd NFs/C60-NH2 (Fig. 1A) demonstrated coating densely of Pd NFs on the C60-NH2 surface. Fig. 1B shows high-resolution TEM (HRTEM) image of the Pd NFs/C60-NH2, which revealed Pd nanoflower on the surface of C60 sheet. X-ray diffraction (XRD) spectra of Pd NFs/C60-NH2 was shown in Fig. 1C, XRD revealed (002) planes of C60 peak at 20.0. However, the weak peaks at 40.0, 46.7, and 68.3°, were assigned to the (111), (200) and (220) planes, respectively, of Pd from the Fm3m space group of the face-centered cubic (fcc) structure [11]. It additionally confirmed the presence of Pd nanoparticles in the nanocomposite of Pd NFs/C60-NH2, but the crystal structure of palladium nanoparticles is not obvious. Additionally, comparing with the FT-IR spectra of C60-NH2, the characteristic band –NH of Pd NFs/C60-NH2 is significantly smaller at 3440 cm-1 corresponding to N-H stretching and the band of N-H bending at 1630 and 915 cm-1, which demonstrate the covalent attachment of amine to the surface of C60 (Fig. 1D).

|
Download:
|
| Fig. 1. (A) TEM image, (B) HRTEM image and (C) XRD patterns of Pd NFs/C60-NH2 nanocomposites. (D) The FT-IR spectras of C60-NH2 (a) and (b). | |
The catalytic performances of Pd NFs/C60-NH2 were first evaluated through the reduction reaction of 4-nitrophenol (NP) to 4-aminophenol (AP) in the presence of NaBH4. Because both the 4-NP and the 4-AP have characteristic UV–vis absorption, it is readily easy to monitor the progress of the reaction by an UV–vis spectrophotometer. Without a catalyst, the reduction reaction, however, cannot proceed (Fig. 2A).The UV–vis absorption peak of 4-nitrophenol (4-NP) appears at λmax =316 nm. 4-Nitrophenolate ion was formed after the addition of NaBH4, which could be found by the red shifted absorption peak to 400 nm [12]. After addition of Pd NFs/C60-NH2, the reaction was initiated. The time-dependent UV–vis absorption spectra were recorded to monitor the process of this reaction. The absorption peak at 400 nm decreased successively (Fig. 2B). At the same time, a new absorption peak at around 306 nm appeared by originated from 4-AP, indicating that 4-NP was reduced to 4-AP. It could be observed that its intensity diminished at the later stage of the reaction. As shown in Fig. 2C, according to the linear relationship of ln(Ct/C0) with t, which agrees with first-order reaction kinetics. The rate constant of the reactions can be estimated for the Pd NFs/C60-NH2 catalysts. It is determined to be 1.805 min-1. The conversion rate in the presence of Pd NFs/ C60-NH2 was much fast, which could be attributed to (i) the big surface area of nanoflowers, (ii) three-dimensional Pd nanostructure and (iii) the synergistic effect between the Pd nanoflower and the C60-NH2 support.

|
Download:
|
| Fig. 2. The time-dependent UV–vis of 4-NP reduction with NaBH4 in the absence (A) and presence (B) of the catalysts Pd NFs/C60-NH2. The plot of ln(Ct/C0) versus reaction time for the 4-NP reduction by catalysts (C). | |
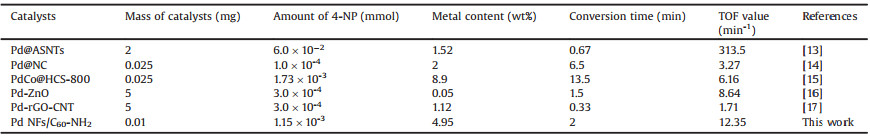
The presence of C60-NH2 can promote to form Pd nanoflower shape and enhance the adsorption of reactant molecules onto the catalytic sites of the Pd nanoparticles through the π–π stacking and electrostatic interaction. Moreover, the structure of Pd NFs/ C60-NH2 has no change after the catalytic reaction, which also indicates that the attachment between Pd NFs and C60-NH2 is sufficiently strong. Herein the catalytic activity of the Pd catalyst was also estimated by turnover frequency (TOF). As shown in Table 1, the Pd NFs/C60-NH2 gives a remarkable TOF of 12.35 min -1 at room temperature. Compared to other similar composites containing Pd-based catalysts [13-17], Pd NFs/C60-NH2 also shows higher catalytic activity for the reaction of reducing 4-NP, suggesting that the self-assembled Pd nanoflowers on C60-NH2 support is an efficient approach for the preparation of high-performance metal nanocatalysts. For practical applications, the reusability of the catalyst has been studied. As shown in Fig. 3, the conversion of 4-NP could keep over 91% after eight cycles, which exhibits excellent recyclability of the Pd NFs/C60-NH2 catalyst.
|
|
Table 1 Comparison of catalytic performance for 4-NP with Pd NFs/C60-NH2 and other catalysts reported. |

|
Download:
|
| Fig. 3. recyclability measurement of Pd NFs/ C60-NH2 during eight successive cycles. | |
In summary, the novel catalyst was prepared by self-assembled palladium nanoflowers supported on amino-functionalized fuller-ene (C60-NH2). The Pd nanocomposite exhibited high catalytic activity and stability toward 4-nitrophenol, which will significant-ly promote catalytic performance due to their ideal morphology.
AcknowledgmentThis work was financially supported by the Natural Science Foundation of Shanxi Province, China (No. 201801D121042).
| [1] |
X.K. Kong, Z.Y. Sun, M. Chen, C.L. Chen, Q.W. Chen, Energy Environ. Sci. 6 (2013) 3260-3266. DOI:10.1039/c3ee40918j |
| [2] |
H. Zhang, Q.Q. Ji, L.D. Lai, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [3] |
S. Kubendhiran, R. Sakthivel, S.M. Chen, B. Maharani, T.W. Chen, Anal. Chem. 90 (2018) 6283-6291. DOI:10.1021/acs.analchem.8b00989 |
| [4] |
Y. Wang, Q.C. Li, P. Zhang, et al., J. Colloid Interf. Sci. 539 (2019) 161-167. DOI:10.1016/j.jcis.2018.12.053 |
| [5] |
L.L. Hu, F. Peng, D.H. Xia, et al., ACS Sustain. Chem. Eng. 6 (2018) 17391-17401. DOI:10.1021/acssuschemeng.8b05169 |
| [6] |
B.Z. Zheng, X.X. Liu, D. Xiao, et al., Inorg. Chem. Front. 4 (2017) 1268-1272. DOI:10.1039/C7QI00262A |
| [7] |
B. Lang, H.K. Yu, Chin. Chem. Lett. 28 (2017) 417-421. DOI:10.1016/j.cclet.2016.10.019 |
| [8] |
K. Gu, X.T. Pan, W.W. Wang, et al., Small 14 (2018) 1801812. DOI:10.1002/smll.201801812 |
| [9] |
L. Zhang, K. Doyle-Davis, X.L. Sun, Energy Environ. Sci. 12 (2019) 492-517. DOI:10.1039/C8EE02939C |
| [10] |
H.J. Wang, L.J. Bai, Y.Q. Chai, R. Yuan, Small 10 (2014) 1857-1865. DOI:10.1002/smll.201303594 |
| [11] |
Z.P. Li, M.N. Ruan, L.H.W. Li, et al., J. Electroanal. Chem. 805 (2017) 47-52. DOI:10.1016/j.jelechem.2017.10.015 |
| [12] |
P. Veerakumar, R. Madhu, S.M. Chen, et al., J. Mater. Chem. A 2 (2014) 16015-16022. DOI:10.1039/C4TA03097D |
| [13] |
J. Liu, J.F. Hao, C.C. Hu, et al., J. Phys. Chem. C 122 (2018) 2696-2703. DOI:10.1021/acs.jpcc.7b10237 |
| [14] |
Y. Tang, R. Huang, C. Liu, et al., Anal. Methods 5 (2013) 5508-5514. DOI:10.1039/c3ay40742j |
| [15] |
S. Choi, M. Oh, Angew. Chem. Int. Ed. 58 (2019) 866-871. DOI:10.1002/anie.201812827 |
| [16] |
C. Zhang, S. Govindaraju, K. Giribabu, Y.S. Huh, K. Yun, Sens. Actuators B:Chem 252 (2017) 616-623. DOI:10.1016/j.snb.2017.06.039 |
| [17] |
D.R. Kumar, M.L. Baynosa, J.J. Shim, Electrochim. Acta 246 (2017) 1131-1140. DOI:10.1016/j.electacta.2017.06.116 |
 2020, Vol. 31
2020, Vol. 31