b School of Aeronautics, Shandong Jiaotong University, Ji'nan 250037, China;
c Faculty of Civil and Geodetic Engineering & Institute of Mathematics, Physics and Mechanics, University of Ljubljana, Ljubljana 1000, Slovenia;
d College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, China;
e Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252000, China
Due to the increasing concern on the global energy and environmental issues, titanium dioxide (TiO2) has attracted great interest as the most promising photocatalyst used in solar energy conversion during the past decades [1-5]. However, pure TiO2 can only absorbs the ultraviolet (UV) light, that is, only 5% of sunlight can be utilized, owing to its relatively wide bandgap (3.20 eV), which thwarted its practical applications [6, 7]. Thus, many efforts have been made to modify the electronic band structure of TiO2 in order to enhance the visible light response by constructing hybrid materials through heteroatom doping, such as metal (e.g., Cr) and non-metal (e.g., N, B) doping as well as metal/non-metal co-doping [7-17]. The most commonly used method for deliberate insertion of the dopants into TiO2 nanostructure is the versatile sol-gel method [18, 19]. Although doping has been demonstrated to be an effective way to modify the electronic properties of the bulk TiO2 and significantly narrow the energy gap, the determination of the atom-precise doping structure and the realization of atomic-level structural modulation are still of great challenges [20].
Recently, titanium-oxo clusters (TOCs) have become one of the most appealing topics since it can not only give precise structural information at the atomic level but also act as the ideal molecular models for further theoretical calculations which thus help us fully understand the structure-property correlations and the fundamental mechanism of TiO2 modification [21, 22]. What is more, the clusters stabilized by organic ligands can also serve as the nanosized building blocks for postfunctionalization and construction of organic-inorganic assemblies in practical applications [5, 23-26]. Interestingly, like the bulk TiO2 nanomaterials, metal ion doping of TOCs can mediate the photochemical formation of holes and electrons. Therefore, large variety of metal ions have been incorporated into TOCs, such as alkali-metal ions [27], transition metal ions [20, 28-32], and lanthanum ions [33, 34], etc. Among those various metal ions, transition metal ions show excellent performance for activating TOCs since they can strongly absorb visible lights in a wider spectral range [28, 35-37].
Based on above considerations, we herein chose inexpensive, easily available copper as the metal doping ion to tune the structure of TOC as well as its bandgap and other properties (e.g., magnetism). Despite CuⅡ ion has already been anchored on TOCs, the CuⅡ ions are usually ligated by the outer ligands of TOCs and the doping concentration was also low [28, 35, 38-42]. Herein, we report the synthesis, structure and properties of such a novel Cu4ⅡTi5Ⅳ heterometallic cluster [Ti5Cu4O6(ba)16] (1, Hba = benzoic acid). Based on this cluster, we realized the inner core doping of CuⅡ ions into TOC with high doping concentration that allows close correlations by direct Cu-O-Ti bonding. Solid-state diffuse reflectance spectra studies indicate that copper ion doping can effectively reduce the bandgap of TOC. Moreover, 1 also shows almost purely paramagnetic behavior with the presence of weak antiferromagnetic interactions at low temperatures.
As shown in Scheme 1, the solvothermal reaction of Ti(OiPr)4 with Hba and CuCl in the presence of 5 μL of HCOOH in CH3CN at 80 ℃ for 3 days gave green crystals of Ti5Cu4O6(ba)16 (1), with the yield of about 65% based on Ti(OiPr)4. Thermal gravity analysis (TGA, Fig. S2 in Supporting information) demonstrates that this cluster can be stable up to about 300 ℃. The slight weight loss before can be ascribed to the removal of the solvent molecules. The total mass loss of about 70% up to 800 ℃ results from the decomposition of the outer organic ligands.
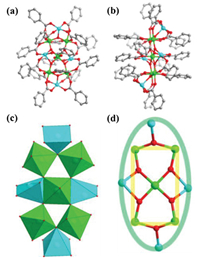
Single crystal X-ray diffraction study shows that 1 crystallizes in triclinic P-1 space group. The asymmetric unit of 1 contains two complete molecules with identical chemical composition for each. Due to the similar structure of two unique clusters, we took only one of them for discussion below. The precise formula of 1 was identified as [Ti5Cu4(μ3-O)6(ba)16] containing five TiⅣ, four CuⅡ, and six μ3-O2- as the core, and sixteen ba- as the ligand shell (Figs. 1a and b), thus the cluster is neutral. All TiⅣ atoms are coordinated by six oxygen atoms forming five octahedra with Ti-O bond of 1.77–2.08 Å; whilst all the CuⅡ are coordinated by five oxygen atoms forming four square pyramids with Cu-O bond lengths in the range of 1.92–2.32 Å (Fig. 1c). The CuO5 square pyramid and TiO6 octahedron connect together using vertex- or edge-sharing mode. The different coordination geometries also provided another evidence to distinguish two different 3d metal atoms. In the Ti5Cu4 core, each CuⅡ atom is bridged by μ3-O2- to connect the other two TiⅣ atoms (Fig. 1d). Five TiⅣ atoms arranged in a rectangle with one of them lying on the center, whereas four CuⅡ atoms encircled Ti5 rectangle, although all CuⅡ atoms are not coplanar with it. Total 16 ba- ligands are found in the same coordination mode of μ2-η1:η1. Among them, two ba- ligands bridge TiⅣ centers, whereas the remaining bridge both CuⅡ and TiⅣ centers. The Cu…Ti and Ti…Ti distances fall in the range of 2.96–3.60 and 3.40–3.54 Å, respectively. Compared to previously reported CuⅡ doped TOCs [38-40, 42], 1 shows very rare bimetal atom arrangement with higher CuⅡ ions doping concentration.
|
Download:
|
| Fig. 1. (a) and (b) Ball-and-stick representation of the molecular structure of 1 viewed along two different orientations. (c) The polyhedral mode of heterometallic Ti5Cu4 core structure. (d) The connection between Ti and Cu atoms. Hydrogen atoms are omitted for clarity. Cu: cyan, Ti: green, O: red, C: grey. | |
|
Download:
|
| Scheme 1. Synthetic Route and the photograph for 1. | |
Solid-state UV–vis absorption spectrum of 1 was measured at room temperature. As shown in Fig. 2a, the absorption bands span from UV region (250–500 nm) to visible-infared region (500– 1100 nm). The absorptions maximum at 356 nm can be primarily attributed to the O 2p → Ti 3d charge transfer (CT) in the core of the cluster, whilst the broader absorption band starting from 500 nm can be ascribed to the d-d transition (2T2g→ 2Eg) of the dopant ion CuⅡ and the penetration of the d electrons into the conduction band (CB) of Ti/O core. [28, 35, 41, 43, 44]. It is worth noting that the absorption in visible region displays strong intensity comparable to that in UV region, which is quite different from the observations of the reported ones [35, 39, 41], where the absorption in visible region is much weaker than that in UV region. Such special absorption behavior may facilitate the utilization of all-range sunlight for photocatalysis. Based on the Kubelka-Munk plot (Fig. 2b), the HOMO–LUMO gap was determined to 2.72 eV, which is lower than that of most reported transition metal doping TOCs [20, 32, 35, 37]. As we know, the bandgap is an essential optical property in coordination compounds, which is influenced by many factors such as metal ions, ligands, bonding behavior between them [45]. We inferred that the narrow bandgap of 1 is likely the result of the unique bimetal connection mode (Cu-O-Ti) in the cluster. What is more, the high doping concentration of CuⅡ that allows more electron injection into the CB of Ti/O core also influenced the electronic structure of 1.

|
Download:
|
| Fig. 2. (a) The solid-state UV–vis spectrum of 1 and (b) the Kubelka-Munk transformation of diffuse reflectance data. | |
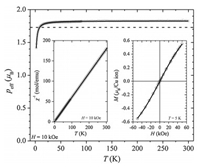
The magnetic properties of the cluster 1 were measured with a Quantum Design MPMS XL-5 magnetometer. The temperature dependence of the magnetic susceptibility χ(T) was measured in external magnetic fields of 1 and 10 kOe both in the zero-field cooled (zfc) and field cooled (fc) regime. There was no splitting between the zfc and fc curves and no difference between the measured susceptibilities in 1 and 10 kOe. Thus, only the fc susceptibility curve measured in 10 kOe is shown for sake of clarity. The magnetization of the sample was measured at 5 K between magnetic field of -50 kOe and 50 kOe. All the presented data were corrected for the contribution of the sample holder and the diamagnetic susceptibility of inner shell electrons [46].
A paramagnetic-like behavior was found on the investigated temperature interval (left inset in Fig. 3). The inverse susceptibility χ-1 was fitted with the Curie-Weiss model, described by the equation χ-1 = (T-θ)/C. The best-fit line is shown as a black line in the left inset in Fig. 3 and was obtained with the fitting parameters C =1.68 emu K/mol and θ = -1.57 K. An effective magnetic moment per Cu ion peff = (8C/4)1/2 = 1.83 μB was obtained, where the factor 4 stands for four CuⅡ ions in the cluster.
|
Download:
|
| Fig. 3. The temperature dependence of peff per CuⅡ ion. The dashed line represents the spin-only theoretical value and the full line the fit with the PHI software. The left inset shows χ-1 as a function of temperature and the fit with the Curie-Weiss model (black line). The right inset shows the M(H) curve with the black curve representing the fit with the Brillouin function. | |
The low temperature magnetization curve M(H) is shown in the right inset of Fig. 3. The curve shows no hysteresis and was successfully fitted with the Brillouin function for the spin value S = 1/2 (black curve in the right inset in Fig. 3). A saturation magnetization Ms = 0.93 μB was obtained from fitting, which is in good agreement with the theoretical value Ms = gS = 1 μB.
Below 20 K the effective magnetic moment starts to decrease slightly and reaches a value of 1.4 μB at the lowest temperature of 2 K. This observation agrees with the calculated negative CurieWeiss temperature θ = -1.57 K. Both experimental results can be ascribed to a single ion effects (for example L-S coupling) and/or weak antiferromagnetic interaction between four Cu(Ⅱ) in a cluster. As the measured high temperature effective magnetic moment of 1.83 μB is quite close to the theoretical spin-only value of 1.73 μB [47], we tentatively contribute the deviation from perfect paramagnetic behavior at low temperatures to weak intramolecular antiferromagnetic interactions.
Based on the structural properties of the cluster a model for the interactions between CuⅡ ions was constructed. It is shown on Fig. 4 and described by the interaction Hamiltonian:

|
Download:
|
| Fig. 4. The model of interactions between CuⅡ ions used in the fitting with the PHI software. | |

|
The experimental data were fitted using the PHI software [48]. Spin-only magnetic moments were assumed, and the only fitting parameters were the coupling constants Ji and the Landé g-factor g. The best-fit line is shown as a black curve on Fig. 3 and was obtained with the fitting parameters J1 = -0.22 cm-1, J2 = -0.21 cm-1, J3 = -1.6 cm-1 and g = 2.1. The reason for a g-value larger than 2 could be the non-zero orbital contribution to the total magnetic moment. The negative coupling constant confirm weak antiferromagnetic interactions between CuⅡ ions.
In magnetic systems which behave almost purely paramagnetic it is hard to distinguish between effect due to interactions between magnetic centers and single ions effects, like spin-orbit coupling. Simulations with the PHI software assuming non-interacting CuⅡ ions and the presence of spin-orbit coupling were performed but did not yield a satisfactory agreement with the measured data for any reasonable choice of parameters. This suggests that the presence of weak antiferromagnetic interactions is indeed the most probable reason for the decrease in peff at low temperatures.
In summary, we have successfully isolated a novel nonanuclear heterometallic with a Ti5Cu4 core stabilized by benzoates. The results reveal that high concentration doping of copper extends the absorption to visible and near-infrared regions with a narrowed bandgap of 2.72 eV. Moreover, the magnetism analysis shows that 1 displays almost pure paramagnetic interactions between CuⅡ ions with the presence of weak antiferromagnetic interactions at low temperatures.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21822107 and 21571115), the Natural Science Foundation of Shandong Province (Nos. BS2014CL042, ZR2017ZF003, JQ201803 and ZR2017MB061), the Taishan Scholar Project of Shandong Province of China, the Qilu Youth Scholar Funding of Shandong University and the Fundamental Research Funds of Shandong University (No. 104.205.2.5), the Slovenian Research Agency (No. P2-0348) and Bilateral Project PR China-Slovenian (No. BI-CN/17-18-004). K. Sheng also acknowledge the State Key Laboratory of Pollution Control and Resource Reuse Foundation (No. PCRRF18019) and financial support from the government of Shandong Province for visiting abroad.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2019.05.050.
| [1] |
X. Chen, L. Liu, P.Y. Yu, S.S. Mao, Science 331 (2011) 746-750. DOI:10.1126/science.1200448 |
| [2] |
J. Schneider, M. Matsuoka, M. Takeuchi, et al., Chem. Rev. 114 (2014) 9919-9986. DOI:10.1021/cr5001892 |
| [3] |
C. Mao, F. Zuo, Y. Hou, X. Bu, P. Feng, Angew. Chem. Int. Ed. 53 (2014) 10485-10489. DOI:10.1002/anie.201406017 |
| [4] |
S. Eslava, A.C. Papageorgiou, S.K. Beaumont, et al., Chem. Mater. 22 (2010) 5174-5178. DOI:10.1021/cm101039k |
| [5] |
G. Fornasieri, L. Rozes, S.L. Calve, et al., J. Am. Chem. Soc. 127 (2005) 4869-4878. DOI:10.1021/ja043330i |
| [6] |
T.L. Thompson, J.T. Yates, Chem. Rev. 106 (2006) 4428-4453. DOI:10.1021/cr050172k |
| [7] |
X. Chen, S.S. Mao, Chem. Rev. 107 (2007) 2891-2959. DOI:10.1021/cr0500535 |
| [8] |
J. Zhu, Z. Deng, F. Chen, et al., Appl. Catal. B:Environ. 62 (2006) 329-335. DOI:10.1016/j.apcatb.2005.08.013 |
| [9] |
M. Pelaez, N.T. Nolan, S.C. Pillai, et al., Appl. Catal. B:Environ. 125 (2012) 331-349. DOI:10.1016/j.apcatb.2012.05.036 |
| [10] |
S.C. Moon, H. Mametsuka, S. Tabata, E. Suzuki, Catal. Today 58 (2000) 125-132. DOI:10.1016/S0920-5861(00)00247-9 |
| [11] |
S.H. Liu, H.R. Syu, Appl. Energy 100 (2012) 148-154. DOI:10.1016/j.apenergy.2012.03.063 |
| [12] |
R. Asahi, T. Morikawa, H. Irie, T. Ohwaki, Chem. Rev. 114 (2014) 9824-9852. DOI:10.1021/cr5000738 |
| [13] |
W. Zhao, W. Ma, C. Chen, J. Zhao, Z. Shuai, J. Am. Chem. Soc. 126 (2004) 4782-4783. DOI:10.1021/ja0396753 |
| [14] |
S. In, A. Orlov, R. Berg, et al., J. Am. Chem. Soc. 129 (2007) 13790-13791. DOI:10.1021/ja0749237 |
| [15] |
S. Ida, N. Kim, E. Ertekin, S. Takenaka, T. Ishihara, J. Am. Chem. Soc. 137 (2015) 239-244. DOI:10.1021/ja509970z |
| [16] |
Z.M. Liu, L. Peng, A.W. Tang, Chin. Chem. Lett. 27 (2016) 1801-1804. DOI:10.1016/j.cclet.2016.04.016 |
| [17] |
Q.P. Liu, Chin. Chem. Lett. 25 (2014) 953-956. DOI:10.1016/j.cclet.2014.03.025 |
| [18] |
G.A. Seisenbaeva, V.G. Kessler, R. Pazik, W. Strek, DaltonTrans. 26 (2008) 3412-3421. DOI:10.1039/B801351A |
| [19] |
Hernandez-Sanchez B.A., T.J. Boyle, C.M. Baros, et al., Chem. Mater. 19 (2007) 1459-1471. DOI:10.1021/cm061798+ |
| [20] |
J.X. Liu, X.C. Zeng, L. Zhang, J. Zhang, Dalton Trans. 45 (2016) 4501-4503. DOI:10.1039/C6DT00333H |
| [21] |
L. Rozes, C. Sanchez, Chem. Soc. Rev. 40 (2011) 1006-1030. DOI:10.1039/c0cs00137f |
| [22] |
P. Coppens, Y. Chen, E. Trzop, Chem. Rev. 114 (2014) 9645-9661. DOI:10.1021/cr400724e |
| [23] |
J. Hou, J. Hu, Q. Sun, et al., Inorg. Chem. 55 (2016) 7075-7078. DOI:10.1021/acs.inorgchem.6b00982 |
| [24] |
S. Trabelsi, A. Janke, R. Hässler, et al., Macromolecules 38 (2005) 6068-6078. DOI:10.1021/ma0507239 |
| [25] |
S. Bocchini, G. Fornasieri, L. Rozes, et al., Chem. Commun. (2005) 2600-2602. DOI:10.1039/b502434j |
| [26] |
L. Rozes, C. Sanchez, Chem. Soc. Rev. 40 (2011) 1006-1030. DOI:10.1039/c0cs00137f |
| [27] |
Y. Chen, E. Trzop, A. Makal, J.D. Sokolow, P. Coppens, Inorg. Chem. 52 (2013) 4750-4752. DOI:10.1021/ic302692d |
| [28] |
S. Eslava, M. McPartlin, R.I. Thomson, J.M. Rawson, D.S. Wright, Inorg. Chem. 49 (2010) 11532-11540. DOI:10.1021/ic101687m |
| [29] |
Y. Lv, J. Willkomm, A. Steiner, et al., Chem. Sci. 3 (2012) 2470-2473. DOI:10.1039/c2sc20193c |
| [30] |
Y. Chen, J.D. Sokolow, E. Trzop, P. Coppens, Dalton Trans. 42 (2013) 15285-15287. DOI:10.1039/c3dt52218k |
| [31] |
C. Artner, A. Koyun, M. Czakler, U. Schubert, Eur. J. Inorg. Chem. 29 (2014) 5008-5014. DOI:10.1002/ejic.201402499 |
| [32] |
Y. Chen, K.N. Jarzembska, E. Trzop, L. Zhang, P. Coppens, Chem. -Eur. J. 21 (2015) 11538-11544. DOI:10.1002/chem.201500961 |
| [33] |
Y. Lv, J. Willkomm, M. Leskes, et al., Chem. -Eur. J. 18 (2012) 11867-11870. DOI:10.1002/chem.201201827 |
| [34] |
S. Wang, H.C. Su, L. Yu, et al., Dalton Trans. 44 (2015) 1882-1888. DOI:10.1039/C4DT02968B |
| [35] |
J.X. Liu, M.Y. Gao, W.H. Fang, L. Zhang, J. Zhang, Angew. Chem. Int. Ed. 55 (2016) 5160-5165. DOI:10.1002/anie.201510455 |
| [36] |
S. Yang, H.C. Su, J.L. Hou, et al., Dalton Trans. 46 (2017) 9639-9645. DOI:10.1039/C7DT01603D |
| [37] |
X.X. Liu, W.H. Fang, S. Chen, L. Zhang, J. Zhang, Chin. J. Chem. 37 (2019) 233-236. DOI:10.1002/cjoc.201800541 |
| [38] |
H. Fang, L. Zhang, J. Zhang, Chem. Commun. 53 (2017) 3949-3951. DOI:10.1039/C7CC01443K |
| [39] |
W.H. Fang, J.F. Wang, L. Zhang, J. Zhang, Chem. Mater. 29 (2017) 2681-2684. DOI:10.1021/acs.chemmater.7b00324 |
| [40] |
L.J. Xu, W.Z. Zhou, L.Y. Zhang, et al., CrystEngComm 17 (2015) 3708-3714. DOI:10.1039/C4CE02505A |
| [41] |
Y.T. Zhang, P. Huang, C. Qin, et al., Dalton Trans. 43 (2014) 9847-9850. DOI:10.1039/c4dt00507d |
| [42] |
J.F. Wang, W.H. Fang, D.S. Li, L. Zhang, J. Zhang, Inorg. Chem. 56 (2017) 2367-2370. DOI:10.1021/acs.inorgchem.6b02913 |
| [43] |
K.N. Jarzembska, Y. Chen, J.N. Nasca, et al., Phys. Chem. Chem. Phys. 16 (2014) 15792-15795. DOI:10.1039/C4CP02509A |
| [44] |
Y.P. He, L.B. Yuan, G.H. Chen, et al., J. Am. Chem. Soc. 139 (2017) 16845-16851. DOI:10.1021/jacs.7b09463 |
| [45] |
B. Zhang, G. Shi, Z. Yang, F. Zhang, S. Pan, Angew. Chem. Int. Ed. 56 (2017) 3916-3919. DOI:10.1002/anie.201700540 |
| [46] |
G.A. Bain, J.F. Berry, J. Chem. Educ. 85 (2008) 532-536. DOI:10.1021/ed085p532 |
| [47] |
J. Coey, Magnetism and Magnetic Materials, Cambridge University Press, New York, 2009.
|
| [48] |
N.F. Chilton, R.P. Anderson, L.D. Turner, A. Soncini, K.S. Murray, J. Comput. Chem. 34 (2013) 1164-1175. DOI:10.1002/jcc.23234 |
 2020, Vol. 31
2020, Vol. 31 

