b Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China
Magnetic Ionic Liquids (MILs) are room-temperature ionic liquids (ILs) [1, 2] that are typically composed of large organic cations and small metal-containing anions, including Fe, Co, Mn, and lanthanide elements [3-5]. They combine magnetic [6] and intrinsic properties of ILs with high thermal stability, low vapor pressure and high conductivity [7]. They have attracted much attention because of their unique paramagnetic properties and potential applications. For example, MILs can be applied in the extractive desulfuration of fuel oils [8], hydrocarbon [4] and the separation of magnetic nanoparticles [9].
The physicochemical behavior of MILs is extremely sensitive to an applied external magnetic field. The relationship between viscosity and the application of external magnetic fields has been investigated [10]. For example, Daniel et al. found the viscosity of [P66614][FeCl4] and [P66614][Cl] mixtures ([P66614]: Trihexyl hexadecyl ammonium) decreased with magnetic field strength [11]. Changes in external conditions, such as pressure, also has a strong influence on the magnetic coupling behavior of ILs, varying from antiferromagnetic to ferrimagnetic ordering [12]. The physicochemical behavior of MILs strongly depends on the interactions between cations and anions and structural composition. Most recent research efforts have focused on intermolecular interactions in MILs. These interactions include dipole-dipole interactions and hydrogen bonds within crystal and molecular structures [13]. Yukihiro et al. acquired infrared and ultravioletvisible absorption spectra to investigate the influence of alkyl chain length and the halide type on the physicochemical properties. Their results revealed that the elongation of the alkyl chain reduces fluidity and ionic conductivity. This effect is mainly ascribed to Van deer Waals attraction between chains. In addition, the replacement of chloride with bromide increases molecular weight and interionic interactions [14]. Similarly, other researchers have indicated that short chain lengths enhance the efficiency of magnetic interaction transmissions [15, 16]. Wang et al. [17] collected Raman and IR spectra to evaluate the low critical solution temperature-type phase behavior of sixteen MILs in water. They confirmed that phase behaviors are strongly dependent on the cation/anion type of MILs.
The physicochemical properties of MILs at room temperature have been extensively investigated, and those of MILs at varying temperatures have also been reported. The density and viscosity of MILs decrease with increments in temperature [18, 19]. Pedro et al. reported on the physical properties of [Emim][FeCl4] ([Emim]: 1-ethyl-3-methylimidazolium) MILs and illustrated the existence of long-range antiferromagnetic ordering below the Néel temperature (TN) of approximately 3.8 K [20]. James et al. examined the resonant gamma ray fluorescence (Mössbauer effect, ME) of 4-piperidinylpyridinium [FeCl4]- at low temperatures and verified the existence of magnetic saturation below 2 K [21]. Likewise, tetraethylammonium tetrachloroferrate(Ⅲ) MILs have been investigated through temperature-dependent Mössbauer spectroscopy. These MILs become antiferromagnetic at a TN of 3.0 K [22]. Herber et al. performed temperature-dependent Mössbauer spectroscopy and XRD to study the dynamic and single-crystal parameters of Felabeled tetrachloroferrate-based MILs [23]. Nevertheless, reliable experimental methods for characterizing the microstructure of MILs remain lacking. In particular, the temperature dependence of the structural evolution of MILs has not been reported in detail.
X-ray absorption fine structure (XAFS) is a highly suitable technique for obtaining the microstructural information of ILs on the atomic level, given its rapid development. Zou et al. [24] have employed XAFS to study the components and structures of ChCl-ZnCl2 ILs with different ChCl:ZnCl2 molar ratios. Jiang et al. [25] performed in-situ XAFS to reveal temperature-induced molecular rearrangement of an ionic liquid confined in nanospaces. Carmichael et al. [26] applied XAFS to elucidate the structures of [C14mim]2[NiCl4] ([C14mim]: 1-tetradecyl-3-methylimidazolium) and [Emim]2[NiCl4] ILs in a boron nitride matrix. The said structure consists of a Ni central atom with four coordination Cl atoms. In this work, the direct microstructural information of [Bmim]FeCl4 MILs at varying temperatures were investigated by using in situ XAFS combined with Raman spectroscopy and DFT calculations.
1-Butyl-3-methylimidazolium ferric tetrachloride ([Bmim]FeCl4) MILs were purchased from Shanghai Chengjie Chemical Co., with 99.0% purity. The MILs were purified carefully in our laboratory by recrystallization, and the purity was confirmed by 1H NMR and HPLC.
The microstructure of [Bmim]FeCl4 MILs at different temperature were characterized by using Raman spectra, XAFS measurement and DFT calculation. Micro-Raman spectra were obtained using a Raman spectrometer (Horiba JY Lab Raman HR 800) with backscattering configuration at different temperature. The laser power of the sample was approximately 80 mW to obtain sufficiently strong Raman signal. Raman spectra were excited by a 532.0 nm laser at a resolution of 0.35 cm-1 (1800 gooves/mm) between 200 cm-1 and 500 cm-1. XAFS measurement was recorded at the Fe K-edge (7112 eV) in transmission mode using a Si(Ⅲ) double-crystal monochromator at the beamline BL14W1 of Shanghai Synchrotron Radiation Facility (SSRF). During measurements, the synchrotron was operated at 3.5 GeV and a current at 210 mA. The photon energy was calibrated using the first inflection point of the Fe K-edge in Fe foil. The K range was from 3.1 Å-1 to 13.5 Å-1, with phase shift correction applied. The (K) data was weighted by K-2. XAFS data was analyzed by the Athena and Artemis programs with the theoretical standards calculated using FEFF8 [27]. DFT calculations were performed with the Gaussian 09 program using density functional theory (DFT) at B3LYP [28] level with LANL2DZ [29] and 6-311G** [30] basis sets. LANL2DZ basis set is being widely utilized in the investigation on transition metal to calculate the equilibrium geometries of Fe(Ⅲ) complexes. 6-311G** basis sets were used for chlorine atoms. The frequency calculations were employed to confirm the resulting geometry as minima.
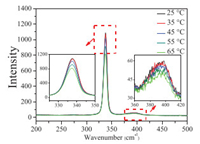
Raman spectra of [Bmim]FeCl4 MILs at different temperatures were carried out and results were shown in Fig. 1. The strong feature peaks at 337 and 396 cm-1 were assigned to the completely symmetrical Fe-Cl stretching vibration of [FeCl4]- in the examined MILs, and correspond well with previously reported values [31, 32]. Feature peaks at 260, 360, and 420 cm-1 ascribable to [Fe2Cl7]- were absent [32-34]. The absence of these peaks indicate that anions in [Bmim]FeCl4 MILs were mainly [FeCl4]- without [Fe2Cl7]- species. The gradual decrement in intensity of the two feature peaks at 337 and 396 cm-1 in the Raman curve with increased temperature implies the structural change of [FeCl4]- species in the anion of [Bmim]FeCl4 MILs.
|
Download:
|
| Fig. 1. Raman spectra of [Bmim]FeCl4 MILs at different temperatures. | |
Raman spectroscopy results verify that temperature variation has a critical effect on the anion structure of [Bmim]FeCl4 MILs. Nevertheless, detailed microstructural information on anion composition remains incompletely. Temperature-dependent XAFS experiments were performed to obtain the details, including the coordination number (CN), atomic distance and type of surrounding atoms, of the local coordination environment that surrounds the Fe atom in [Bmim]FeCl4 MILs. The results are discussed below.
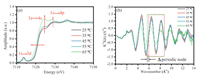
The Fe K-edge XANES spectra and the corresponding Fe K-edge EXAFS oscillation of [Bmim]FeCl4 MILs at different temperatures (25 ℃, 35 ℃, 45 ℃, 55 ℃, 65 ℃) are given in Fig. 2. XANES data was subjected to background subtraction and normalization. Fig. 2a shows that Fe K-edge XANES obtained at different temperatures exhibited a wide variety of pre-edge feature peaks at 7114 eV. These peaks originated from a 1s → 3d transition [35]. Spin state, oxidation state and geometry are strongly influenced by energy position and intensity [36]. The 1s → 3d transition is partly forbidden for a centrosymmetric Fe site, such as that in an octahedral coordination. A pre-edge feature peak due to quadrupole coupling remained visible in the experimental spectra [37]. There is no significant variation in the position of the pre-edge peak at 7114 eV with increments in temperature, indicated that the Fe3+ has not been reduced to Fe2+ (average pre-edge position 7112 eV) [38]. The intensity of the pre-edge peak decreased, and the intensities of the peaks at 7124 and 7129 eV, which were ascribed to the 1s → 4s and 1s → 4p transitions, respectively, increased as the temperature was increased. These behaviors indicate that temperature affected the microstructure of [Bmim] FeCl4 MILs. The variations in the intensity and position of the white line peak at 7124 eV at temperatures up to 65 ℃ imply remarkable structural changes. Fig. 2b shows the corresponding Fe K-edge EXAFS oscillation of [Bmim]FeCl4 MILs at different temperatures. The reduction in amplitude with the elevation in temperature validates the reduction in the anion-cation CN. Additionally, the extension of the periodic node at temperatures up to 65 ℃ indicates shortened anion-cation distances.
|
Download:
|
| Fig. 2. Fe K-edge XANES spectra (a) and corresponding Fe K-edge EXAFS oscillation (b) of [Bmim]FeCl4 MILs at different temperatures. | |
The Fe K-edge XAFS data of [Bmim]FeCl4 MILs at different temperatures were fitted to extract Fe-Cl CN and bond length (R) to obtain the temperature-dependent effects on the structure of MILs. The Fe-Cl scatter path was used to fit the Fe K-edge XAFS data for [Bmim]FeCl4 MILs at all temperatures. The R-space experimental results and fitting data for [Bmim]FeCl4 MILs at different temperature are shown in Fig. S1 (Supporting information). The fitting structural parameters are summarized in Table 1.
|
|
Table 1 Structural parameters of [Bmim]FeCl4 MILs obtained through in-situ Fe K-edge XAFS experiments at different temperatures. Fitting was conducted in R-space by using quick first shell theory (Fe-Cl) and K1, 2, 3 weighting. 1.2 < R < 2.2 Å and △ K = 3.1– 13.5 Å -1 were used for Fe. |
As shown in Fig. S1, the appearance of a feature peak at approximately 1.82 Å (phase shift uncorrected) with all temperatures is attributed to Fe-Cl background scatter. Fig. S1 reveals that the amplitude of Fe-Cl background scatter reduced with increments in temperature. This reduction indicates that the CN of the Cl atom around the Fe atom decreased. This result is consistent with the XANES analysis, and can also be reflected by the fitting parameter (Table 1). A careful analysis of Table 1, revealed that the average CN of Fe-Cl was 3.3 ± 0.4 and the average R of Fe-Cl was 2.21 Å. These results verify the existence of tetrahedral [FeCl4]- at 25 ℃ and 35 ℃. The average Fe-Cl CN of 2.8 ± 0.4 and the average of Fe-Cl R of 2.20 Å may indicate the existence of bridge-chain [Fe2Cl5]+ with three coordination Cl around Fe at 45 ℃ and 55 ℃. The [FeCl4]- anion in [Bmim]FeCl4 MILs may induce dissociation to yield FeCl3, in accordance with the equation [FeCl4]-⇌FeCl3 + Cl-. Then, the combination of two FeCl3 likely yielded [Fe2Cl5]+ in accordance with eq. (2) FeCl3 ⇌ [Fe2Cl5]+ + Cl-. Similarly, Melissa et al. [32] revealed that [Fe2Cl7]- appear with excess FeCl3 in [Bmim]Cl ILs by Raman scattering and ab initio calculations. Li et al. [39] found that the dimer structure [Pt2Cl8]2- formed in [Bmim]2PtCl4 ILs confined in silica nanopores. However, the CN of 1.8 ± 0.2 and the R of 2.17 Å of Fe-Cl possibly indicate the appearance of [FeCl2]+ with two coordination Cl around Fe at 65 ℃. The obtained FeCl3 may dissociate to form [FeCl2]+ as represented by the following equilibrium equation: FeCl3 ⇌ [FeCl2]+ + Cl-. Therefore, the reductions in Fe-Cl CN (from 3.3 to 1.8) and in Fe-Cl R from 2.21 Å to 2.17 Å observed through XAFS are indicative of the dissociation of tetrahedral [FeCl4]- and the formation of bridgechain [Fe2Cl5]+, and even [FeCl2]+ species with increasing temperature. These results are in agreement with Raman and Fe K-edge EXAFS oscillation results, and indicate that dissociation is an endothermic reaction, that is promoted by elevated temperature. Scheme S1 (Supporting information) shows the proposed structure of the anion of [Bmim]FeCl4 MILs at different temperatures.
XAFS results revealed that CN decreased and Fe-Cl R shortened in [Bmim]FeCl4 MILs with increments in temperature. The XAFS results indicated that the different anions in [Bmim]FeCl4 MILs generated the following species: (1) anions with four coordination Cl atoms around the Fe atom with a tetrahedral structure [FeCl4]-; (2) three Fe-Cl CN with a bridge-chain [Fe2Cl5]+ species; (3) FeCl2+ species with two Fe-Cl CN. DFT calculations for the above [FexCly]3x-y species were performed to determine the correlation between CN and Fe-Cl R. The results are shown in Fig. S2 (Supporting information). The structural parameters are summarized in Table 2. The DFT calculations shown in Table 2 confirm that the R of Fe-Cl decreased from 2.19 Å to 2.06 Å as the CN of Fe-Cl decreased from four to two coordination Cl ions around Fe. This finding is in excellent agreement with the XAFS results.
|
|
Table 2 Average Rs of Fe-Cl for the different [FexCly]3x-y species obtained through DFT calculations. |
In summary, the temperature-dependent structural changes of [Bmim]FeCl4 MILs were investigated through in-situ XAFS combined with Raman spectroscopy and DFT calculations. Raman spectroscopy indicated that the amount of [FeCl4]- species decreased with increments in temperature. In-situ XAFS directly revealed that the reduction in the CN and the R of Fe-Cl in the anion of [Bmim]FeCl4 MILs with elevated temperature, could be ascribed to the dissociation of tetrahedral [FeCl4]-, and the formation of bridge-chain [Fe2Cl5]+, and [FeCl2]+ species. These results showed that dissociation reaction was an endothermic, and was promoted by elevated temperatures. DFT calculations further showed that the R of Fe-Cl gradually decreased as the CN of Fe-Cl decreased. The results of this work may provide a detailed theoretical basis for understanding the structural changes shown by MILs as result of temperature-dependent effects.
AcknowledgmentsThis work was sponsored by the National Natural Science Foundation of China (Nos. 21802095, 11805261), Shanghai Sailing Program (No. 19YF1458200), the China Postdoctoral Science Foundation (No. 2017M621468) and the Joint Funds of the National Natural Science Foundation of China (No. U1832152). XAFS experiments were carried out at beamline BL14W1 SSRF (Shanghai Synchrotron Radiation Facility).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2019.05.028.
| [1] |
T. Welton, Chem. Rev. 99 (1999) 2071-2083. DOI:10.1021/cr980032t |
| [2] |
E. Santos, J. Albo, A. Irabien, RSC Adv. 4 (2014) 40008-40018. DOI:10.1039/C4RA05156D |
| [3] |
A. Branco, L.C. Branco, F. Pina, Chem. Commun. 47 (2011) 2300-2302. DOI:10.1039/C0CC03892J |
| [4] |
S.A. Sakal, Y.Z. Lu, X.C. Jiang, et al., J. Chem. Eng. Data 59 (2014) 533-539. DOI:10.1021/je400076x |
| [5] |
K.D. Clark, O. Nacham, H.L. Yu, et al., Anal. Chem. 87 (2015) 1552-1559. DOI:10.1021/ac504260t |
| [6] |
S. Hayashi, H.O. Hamaguchi, Chem. Lett. 33 (2004) 1590-1591. DOI:10.1246/cl.2004.1590 |
| [7] |
Y. Yoshid, J. Fujii, K. Muroi, et al., Synthetic Met. 153 (2005) 421-424. DOI:10.1016/j.synthmet.2005.07.321 |
| [8] |
N.H. Ko, J.S. Lee, E.S. Huh, et al., Energy Fuel. 22 (2008) 1687-1690. DOI:10.1021/ef7007369 |
| [9] |
S.D. Sun, X.L. Li, J. Am. Oil. Chem. Soc. 93 (2016) 757-764. DOI:10.1007/s11746-016-2826-5 |
| [10] |
C.I. Daniel, F.V. Chavez, G. Feio, et al., J. Phys. Chem. B 117 (2013) 11877-11884. DOI:10.1021/jp4078536 |
| [11] |
C.I. Daniel, F.V. Chavez, C.A.M. Portugal, et al., J. Phys. Chem. B 119 (2015) 11740-11747. DOI:10.1021/acs.jpcb.5b04772 |
| [12] |
A. Garcia-Saiz, de Pedro I., J.A. Blanco, et al., J. Phys. Chem. B 117 (2013) 3198-3206. DOI:10.1021/jp3114623 |
| [13] |
J. Dupont, J. Braz. Chem. Soc. 15 (2014) 341-350. DOI:10.1590/S0103-50532004000300002 |
| [14] |
Y. Yoshida, G.Z. Saito, J. Mater. Chem. 16 (2006) 1254-1262. DOI:10.1039/b515391c |
| [15] |
Y. Yoshida, A. Otsuka, G. Saito, et al., Chem. Soc. Jpn. 78 (2005) 1921-1928. DOI:10.1246/bcsj.78.1921 |
| [16] |
Garcia-Saiz A., P. Migowski, O. Vallcorba, et al., Chem. Eur. J. 20 (2014) 72-76. DOI:10.1002/chem.201303602 |
| [17] |
Y.C. Pei, Y. Cao, Y.J. Huang, et al., Sci China-Chem. 59 (2016) 587-593. DOI:10.1007/s11426-016-5577-0 |
| [18] |
E. Santos, J. Albo, A. Rosatella, et al., J. Chem. Technol. Biot. 89 (2014) 866-871. DOI:10.1002/jctb.4323 |
| [19] |
M.M. Cruz, R.P. Borges, M. Godinho, et al., Fluid Phase Equilibr. 350 (2013) 43-50. DOI:10.1016/j.fluid.2013.03.001 |
| [20] |
I. de Pedro, D.P. Rojas, J. Albo, et al., J. Phys. -Condens. Mat. 22 (2010). |
| [21] |
B.D. James, S.M. Juraja, J. Liesegang, et al., Inorg. Chim. Acta 312 (2001) 88-92. DOI:10.1016/S0020-1693(00)00328-5 |
| [22] |
C.S.M. Partiti, H.R. Rechenberg, Hyperfine Interact. 70 (1992) 1075-1078. DOI:10.1007/BF02397515 |
| [23] |
R.H. Herber, I. Nowik, M.E. Kostner, et al., Int. J. Mol. Sci. 12 (2011) 6397-6406. DOI:10.3390/ijms12106397 |
| [24] |
Y. Zou, H.J. Xu, G.Z. Wu, et al., J. Phys. Chem. B 113 (2009) 2066-2070. DOI:10.1021/jp809788u |
| [25] |
F.L. Jiang, C. Li, H.Y. Fu, et al., J. Phys. Chem. C 119 (2015) 22724-22731. DOI:10.1021/acs.jpcc.5b07325 |
| [26] |
A.J. Carmichael, C. Hardacre, J.D. Holbrey, et al., Anal. Chem. 71 (1999) 4572-4574. DOI:10.1021/ac990332q |
| [27] |
J.J. Rehr, R.C. Albers, Rev. Mod. Phys. 72 (2000) 621-654. DOI:10.1103/RevModPhys.72.621 |
| [28] |
C. Lee, W.T. Yang, R.G. Parr, Phys. Rev. B 17 (1998) 785-789. DOI:10.1103/PhysRevB.37.785 |
| [29] |
P.J. Hay, W.R. Wadt, J. Chem. Phys. 82 (1985) 270-284. DOI:10.1063/1.448799 |
| [30] |
M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 09, Revision C.01 Wallingford CT, 2009.
|
| [31] |
H. Wang, R.Y. Yan, Z.X. Li, et al., Catal. Commun. 11 (2011) 763-767. DOI:10.1016/j.catcom.2010.02.011 |
| [32] |
M.S. Sitze, E.R. Schreiter, E.V. Patterson, et al., Inorg. Chem. 40 (2001) 2298-2304. DOI:10.1021/ic001042r |
| [33] |
J.Z. Yang, W.G. Xu, Q.G. Zhang, et al., J. Chem. Thermodyn. 35 (2003) 1855-1860. DOI:10.1016/j.jct.2003.07.002 |
| [34] |
G.N. Papatheodorou, G.A. Voyiatzis, Chem. Phys. Lett. 303 (1999) 151-156. DOI:10.1016/S0009-2614(99)00213-4 |
| [35] |
R.G. Shulman, Y. Yafet, P. Eisenberger, et al., Proc. Natl. Acad. Sci. U. S. A. 73 (1976) 1384-1388. DOI:10.1073/pnas.73.5.1384 |
| [36] |
T.E. Westre, P. Kennepohl, J.G. DeWitt, et al., J. Am. Chem. Soc. 119 (1997) 6297-6314. DOI:10.1021/ja964352a |
| [37] |
R.A. Blair, W.A. Goddard, Phys. Rev. B 22 (1980) 2767-2776. DOI:10.1103/PhysRevB.22.2767 |
| [38] |
M. Wilke, F. Farges, P.E. Petit, et al., Am. Mineral. 86 (2001) 714-730. DOI:10.2138/am-2001-5-612 |
| [39] |
C. Li, Y.X. Wang, X.J. Guo, et al., J. Phys. Chem. C 118 (2014) 3140-3144. DOI:10.1021/jp4115942 |
 2020, Vol. 31
2020, Vol. 31