b School of Chemistry and Food Engineering, Changsha University of Science and Technology, Changsha 410114, China
Since its role as a neuron modulator was first revealed in 1996 [1], hydrogen sulfide (H2S) has been recognized as a unique signalling molecule that exerts protective effects in mammals against a range of conditions and events, such as inflammation [2], Parkinson's disease [3], cancer [4] and dermal wounds [5]. Accordingly, the therapeutic potential of this gasotransmitter has stimulated wide interest in the development of H2S-releasing agents (H2S donors). In this regard, developing a strategy for controlled generation of H2S with high spatiotemporal precision is hence critically important for optimization of the therapeutic effect of H2S [6, 7].
Several types of H2S donors that release H2S in response to stimuli such as external light [8], pH [9], reactive oxygen species [10] and enzymatic activity [11]. have been reported. Among these strategies, light-induced H2S release has gained considerable attention because of its ability to provide spatiotemporal control over H2S release. Furthermore, controlling the period and intensity of the light irradiation can modulate the extent of H2S release and its duration [12, 13]. Photothermal triggering, as one of the light stimuli system, has emerged as a particularly promising technique for controlled release applications because of its biological benignity and convenient operation [14]. Photothermal release typically involves the application of plasmonic nanoparticles (pNPs) [15]. However, to the best of our knowledge, there are no reports on the use of pNPs for photothermally triggering H2S release in specific spatial and temporal patterns.
Dithiocarbamate (DTC) compounds are thermo-labile organosulfur compounds that are capable of H2S generation upon thermal pyrolysis [16]. Accordingly, we reasoned that if DTC compounds were combined with pNPs, the pNPs would convert incident light into heat and trigger the thermal decomposition of the guest molecules. Thus, a new light-driven H2S-releasing platform based on photothermal transduction could be established.
To demonstrate the feasibility of this concept, here we present the use of near-infrared (NIR) light-driven photothermally responsive nanocomposites for H2S release. Our H2S-releasing system consists of thermo-labile polyethylenimine dithiocarbamate (PEI-DTC) polymers as H2S donors and pNPs as NIR photothermal antennae combined in agarose hydrogel. As illustrated in Fig. 1, the dithiocarbamate addition reaction between polyethylenimine (PEI) and carbon disulfide (CS2) results in the formation of a PEI-DTC polymer, which decomposes into H2S at elevated temperature. Au@Ag/Au core@shell alloyed pNPs were used as NIR light nanotransducers owing to their large absorption cross section and efficacious hyperthermia in the NIR window [17]. Under 780 nm NIR photoirradiation, Au@Ag/Au pNPs absorb photoirradiation and converted it to thermal energy, initializing the thermal decomposition of the PEI-DTC polymer donor and thus generating H2S (Fig. 1A). The loading of the photothermal donors in agarose hydrogel allows a spatiotemporally controlled approach for the photothermal generation of H2S at concentrations in the micromolar range. The biological utility of our photothermal H2S-donating system was demonstrated by treating the inflammation of rat toes. Our system uniquely combines the features of the strong photothermal reactivity of pNPs and a thermo-labile H2S polymer donor. The ability to modulate the photoirradiation for controlled generation and spatiotemporally release of H2S are its specific advantages.
|
Download:
|
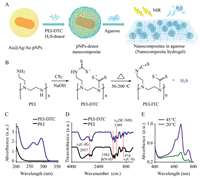
| Fig. 1. Photothermally controlled generation of H2S from nanocomposite agarose hydrogels. (A) PEI-DTC polymer H2S donors are adsorbed on the surface of Au@Ag/Au pNPs and then loaded into agarose hydrogel. NIR photoirradiation triggers the release of H2S through photothermal transduction. (B) Schematic of PEI-DTC preparation and its thermal decomposition. Decomposition of the monoalkyl dithiocarbamate fragment at 30-200 ℃ generates of H2S. (C) UV–vis absorption spectra and (D) FTIR spectra of PEI-DTC and PEI polymers. (E) Methylene blue colorimetric analysis of H2S generated from the thermal decomposition of PEI-DTC at 45 ℃ for 350 min (20 ℃ employed as a control temperature). | |
Our fabrication of the thermo-labile H2S-donor polymer PEI-DTC is based on dithiocarbamate condensation of CS2 with the primary and secondary amine groups in the branched PEI polymer under basic conditions (Fig. 1B) [18]. The reaction of the PEI with excess CS2 (CS2/PEI = 1:8, w/w) at pH 7.0~9.0 yielded the PEI–DTC polymer as the major product (>90%). The photophysical properties of this polymer were investigated. Two new absorption peaks at 260 and 290 nm, which are characteristic absorptions of dithiocarbamate, are observed (Fig. 1C) [19]. The formation of PEI–DTC neutralized the positive charge of the polymer from 15.3 ± 1.2 mV to 4.9 ± 1.3 mV (Fig. S1 in Supporting information). The formation of PEI–DTC was further confirmed by FTIR and 13C NMR. The FTIR spectrum of PEI–DTC presents new bands at 1389 cm-1 and 974 cm-1, which are characteristic bending vibrations of the CS–NH bonds and C–S bonds in the dithiocarbamate, respectively (Fig. 1D). Compared with the 13C NMR spectrum of PEI, a new chemical shift at 211 ppm appears in that of PEI–DTC, which is assigned to the dithiocarbamate (Fig. S2 in Supporting information). Thus, the above results confirm the formation of PEI–DTC, consistent with our previous results [20].
We next examined the thermal decomposition of the PEI-DTC H2S donor using thermogravimetric analysis (TGA). PEI-DTC powder exhibits rapid weight loss in the temperature ranges 30~200 ℃ and 240~390 ℃ (Fig. S3 in Supporting information)). Monoalkyl dithiocarbamates were reported to exhibit high thermal labilities [21]. The weight loss in the temperature range (30~200 ℃), which corresponds to decomposition of the primaryamine-derivatized monoalkyl dithiocarbamate fragment, is likely to be responsible for the generation of H2S (Fig. 1D). To verify this, the PEI-DTC was heated at 45 ℃ for 530 min (Fig. S4 in Supporting information)) and the pyrolytic products were collected for H2S determination, which was performed using a standard methylene blue assay (Fig. S5 in Supporting information)) [22]. A remarkable increase in colorimetric adsorption at 667 nm was observed compared with that of the control (i.e., the product mixture collected at 20 ℃) (Fig. 1E), which clearly confirms that the elevated temperature facilitates the thermal decomposition of PEI-DTC and the generation of H2S. In our system, the largest amount of H2S released from the polymer donor was 33.2 μg from 1.0 mg PEI-DTC at 100 ℃ for 4 h (Fig. S6 in Supporting information)).
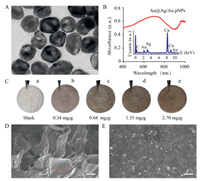
Having confirmed that the thermal decomposition of PEI-DTC provides H2S, we sought to devise a photothermally responsive system in which a H2S donor is combined with pNPs and loaded into agarose hydrogel. The Au@Ag/Au pNPs were prepared according to our previous protocol [17]. Transmission electron microscopy (TEM) and energy dispersive X-ray spectrometry (EDS) confirmed the successful preparation of the pNPs, which were observed to have an average diameter of 40.2 ± 5.7 nm and a coreshell structure morphology with a Au nanorod core and a Ag/Au alloy shell (Fig. 2A and inset of Fig. 2B). The nanoparticles exhibit strong UV-vis-NIR plasmonic adsorption in the wavelength range 400-1000 nm, indicative of pNPs that exhibit a strong photothermal response (Fig. 2B). The PEI-DTC was adsorbed onto the pNPs through electrostatic adsorption or coordination interaction, leading to the formation of donor-pNPs nanocomposites. Saturated adsorption of the PEI-DTC polymer onto the pNPs reverses their zeta potential, which changes from -18.4 ± 2.0 mV to 16.0 ± 2.4 mV (Fig. S7 in Supporting information). Hydrodynamic size of Au@Ag/Au pNPs was determined by dynamic light scattering (DLS), which revealed that their size increased from 43.6 ± 3.3 nm to 52.5 ± 2.5 nm after coating of PEI-DTC polymer (Fig. S8 in Supporting information). A maximum loading capacity of 1.12 mg PEI-DTC per mg of pNPs at room temperature was determined through a Langmuir equilibrium isotherm experiment (Fig. S9 in Supporting information).
|
Download:
|
| Fig. 2. (A) TEM image, (B) UV–vis absorption spectrum, and EDS analysis of Au@Ag/ Au pNPs (inset). (C) Hydrogels loaded with different amounts of nanocomposite. (D) SEM image of a nanocomposite hydrogel. (E) Magnification of the red rectangle in (D). Scale bar: 20 nm (A), 50 μm (D) and 400 nm (E). | |
The encapsulation of the nanocomposites into biologically inert agarose hydrogel would allow spatiotemporally localized gas release, which would be highly convenient for biomedical applications [23, 24]. With this in mind, we loaded the donorpNPs nanocomposites into agarose hydrogel. Fig. 2C shows five nanocomposite hydrogels loaded with different amounts of the pNPs-polymer nanocomposite (0~2.70 mg/g). The loading of nanocomposites causes a colour change in the hydrogel from white to brown. The morphology of the nanocomposite hydrogel was investigated by SEM (Fig. 2D). The nanocomposite hydrogel is assembled from thin wrinkled layers with a spatial network structure, and the layers are decorated with pNPs. As illustrated by the magnified image in Fig. 2E, the pNPs distributed on the hydrogel layers are estimated to be 45.0 ± 6.2 nm in diameter and are therefore likely to be the pNPs.
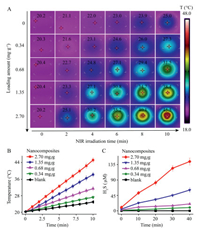
The photothermal properties of the nanocomposite hydrogels were investigated through photothermal imaging experiments. As shown in Fig. 3A, the hydrogel temperature gradually increases with illumination time, with an increase in nanocomposite loading facilitating temperature elevation. The quantitative photothermal responsive curves shown in Fig. 3B demonstrate that a nanocomposite loading of 2.70 mg/g results in a temperature increase from 20.2 ℃ to 45.5 ℃ upon NIR illumination for 10 min.
|
Download:
|
| Fig. 3. (A) Thermal images of nanocomposite hydrogels loaded with different amounts of nanocomposite (0~2.70 mg/g) upon NIR (3.18 W/cm2) illumination for different durations. (B) Quantitative temperature change plot of the data presented in (A). (C) Time-dependent H2S generation from hydrogels loaded with different amounts of nanocomposite (0~2.70 mg/g). | |
The photothermal effect facilitates the thermal decomposition of the polymer donor and the generation of H2S from the nanocomposite hydrogel. After NIR illumination, strong fluorescence is observed from a nanocomposite hydrogel stained with the H2S fluorescence probe 7-azido-4-methylcoumarin (AzMC) (Fig. S10 in Supporting information), which confirms the photothermal generation of H2S in the hydrogels. Colorimetric quantitative analysis showed that the amount of H2S released from the hydrogel gradually increases with illumination time, and higher nanocomposite loading facilitates the photogeneration of H2S (Fig. 3C and Fig. S11 in Supporting information), which is consistent with the fluorescent hydrogel imaging results (Fig. S12 in Supporting information). The agarose hydrogel loaded with nanocomposite at 2.70 mg/g exhibits an increase in H2S concentration to 144.0 μmol/L under 780 nm laser illumination for 40 min (Fig. 3C).
In our system, a H2S-releasing polymer donor, photothermal pNPs, and light illumination are the three essential requirements for the photothermal generation of H2S. Removal of one of these components stops the generation of H2S, as confirmed by control experiments (Figs. S13 and S14 in Supporting information). NIR photoirradiation of the hydrogel containing both NPs and the H2S donor leads to the generation of H2S at 82.0 μmol/L, which is far higher than that by the hydrogel in the absence of pNPs (36.8 μmol/L) (Fig. S13). The results also provide compelling evidence that the photothermal pNPs are largely responsible for the generation of H2S.
The prime advantage of this photothermal H2S-releasing system is the convenient modulation of the excitation illumination for H2S generation. When the nanocomposite hydrogel is irradiated at different laser powers, H2S generation is modulated accordingly. For example, when nanocomposite irradiation at 3.18 W/cm2 is stopped after 15 min, the H2S production rate decelerates from 2.41 μmol L-1 min-1 to 0.12 μmol L-1 min-1 over 50 min. However, the production rate is restored to 0.93 μmol L-1 min-1 when laser excitation is restarted (Fig. S15 in Supporting information). Highpower laser photon illumination facilitates the rapid generation of H2S. For example, when the excitation power is increased stepwise from 0.06 W/cm2 to 3.60 W/cm2, the H2S generation rate increases from 0.8 μmol L-1 min-1 to 2.3 μmol L-1 min-1 (Fig. S15). Thus, our system provides a facile and versatile platform for tuneable and spatiotemporally controlled H2S release using NIR laser irradiation.
Because H2S can induce neutrophil apoptosis and contribute to anti-inflammation [25], we further evaluated the ability of our photothermal H2S-releasing platform to alleviate inflammation under NIR irradiation. The anti-inflammation experiment was conducted based on rat toe volume measurements (Fig. 4A) [26]. Typically, egg albumin was injected into a rat toe to induce local inflammation. Egg albumin injection causes edema in the toe of the rat and an emergence of swelling, indicative of the occurrence of inflammation (Fig. 4B). Then, a nanocomposite hydrogel (loading content 2.70 mg/g) was applied to the swollen area of the toe and subjected to NIR irradiation. A polymer donor-free agarose hydrogel was used as a control (Fig. S16 in Supporting information). Nanocomposite hydrogel has good biosafety, and no obvious cytotoxicity was observed for treatment with cells, which was proved by cell viability tests to quantitatively test the cytotoxicity of the nanocomposite hydrogel (Fig. S17 in Supporting information).
|
Download:
|
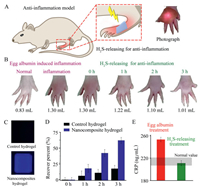
| Fig. 4. In vivo anti-inflammation evaluation of nanocomposite hydrogel treatment. (A) Schematic of the rat model employed. (B) Rat toes in different conditions (normal, egg albumin treatment, and following H2S-releasing treatment for different time intervals). (C) Fluorescence detection of the photothermal generation of H2S in a hydrogel using an AzMC probe. (D) Recovery percent of the rat toe volumes under H2S releasing treatment. (E) Quantitative expression of CRP in rat blood before and after treatment with nanocomposite hydrogels. | |
Photothermal generation of H2S was confirmed by an AzMC fluorescent probe, which presented a strong blue fluorescence in the nanocomposite hydrogel but no discernible fluorescence in the H2S-donor-free control hydrogel (Fig. 4C). After H2S-releasing treatment, the volume of the swollen toe gradually decreased (Fig. 4B), as evaluated by measuring the volume change of the rat toe (Fig. S18 in Supporting information). As shown in Fig. 4B, photothermal release of H2S alleviates the inflammation, leading to a gradual decrease in the toe volume. After H2S-releasing treatment for 3.0 h, the toe volume decreased from 1.30 mL to 1.01 mL, constituting a recovery percentage value of 62.1%, which is far higher than the value of 17.6% observed for the control hydrogel (Fig. 4D).
In order to confirm that the toe volume change was associated with the alleviation of inflammation, C-reactive protein (CRP), a substance produced by the liver in response to inflammation, was determined through the enzyme-linked immunosorbent assay method. As illustrated in Fig. 4E, the CRP content of the rat blood increases rapidly to 253.2 ± 4.2 ng/mL upon egg albumin treatment. As expected, upon H2S-releasing treatment, the CRP content decreases to 211.0 ± 4.6 ng/mL, which is located in the normal concentration range. Therefore, the results clearly demonstrate that the photothermal H2S-releasing system has effective antiinflammatory functionality.
In summary, a novel NIR-activated H2S-donating nanocomposite hydrogel was developed. This strategy represents the first example of H2S release upon photothermal triggering using pNPs as NIR antennae. PEI-DTC donors were assembled on the pNPs, which were then loaded into agarose hydrogel. The release of H2S could be modulated in terms of spatial and temporal control by changing the irradiation parameters. In addition, the system was demonstrated to be capable of alleviating inflammation in rat toes. This work provides a new approach to the construction of photothermally sensitive H2S-release systems and demonstrates the potential utility of these complexes as innovative drugs for the treatment and prevention of serious medical conditions.
AcknowledgmentsThe authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. 21735002, 21575037, 21778016, 21675046 and 21877030), Natural Science Foundation of Hunan Province (No. 2177JJ3026), Keypoint Research and Invention Program of Hunan Province (No. 2017DK2011).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2019.05.025.
| [1] |
K. Abe, H. Kimura, J. Neurosci. 16 (1996) 1066-1071. DOI:10.1523/JNEUROSCI.16-03-01066.1996 |
| [2] |
M. Li, J. Li, T. Zhang, et al., Eur. J. Med. Chem. 138 (2017) 51-65. DOI:10.1016/j.ejmech.2017.06.012 |
| [3] |
X. Cao, L. Cao, L. Ding, J. Bian, Mol. Neurobiol. 55 (2018) 3789-3799. DOI:10.1007/s12035-017-0617-0 |
| [4] |
Y. Liu, F. Yang, C. Yuan, et al., ACS Nano 11 (2017) 1509-1519. DOI:10.1021/acsnano.6b06815 |
| [5] |
J. Wu, Y. Li, C. He, et al., ACS Appl. Mater. Interfaces 8 (2016) 27474-27481. DOI:10.1021/acsami.6b06466 |
| [6] |
S. Gao, G. Tang, D. Hua, et al., J. Mater. Chem. B 7 (2019) 709-729. |
| [7] |
Z. Xiao, T. Bonnard, A. Shakouri-Motlagh, et al., Chem. -Eur. J. 23 (2017) 11294-11300. DOI:10.1002/chem.201701206 |
| [8] |
S.Y. Yi, Y.K. Moon, S. Kim, et al., Chem. Commun. 53 (2017) 11830-11833. DOI:10.1039/C7CC06990A |
| [9] |
J. Kang, Z. Li, C.L. Organ, et al., J. Am. Chem. Soc. 138 (2016) 6336-6339. DOI:10.1021/jacs.6b01373 |
| [10] |
Y. Zhao, M. Pluth, Free Radic. Biol. Med. 128 (2016) 14858-14862. DOI:10.1016/j.freeradbiomed.2016.05.027 |
| [11] |
C.R. Powell, J.C. Foster, B. Okyere, M.H. Theus, J.B. Matson, J. Am. Chem. Soc. 138 (2016) 13477-13480. DOI:10.1021/jacs.6b07204 |
| [12] |
V. Yarra, J. Das, A. Chaudhuri, et al., Chem. Commun. 54 (2018) 3106-3109. DOI:10.1039/C8CC01172A |
| [13] |
W. Chen, M. Chen, Q. Zang, et al., Chem. Commun. 51 (2015) 9193-9196. DOI:10.1039/C5CC02508G |
| [14] |
J. Chen, C. Ning, Z. Zhou, et al., Prog. Mater. Sci. 99 (2019) 1-26. DOI:10.1016/j.pmatsci.2018.07.005 |
| [15] |
P. Lester, Z. Wesley, H. Dennis, et al., ACS Nano 4 (2010) 6395-6403. DOI:10.1021/nn1016346 |
| [16] |
A.K. Sharma, Thermochim. Acta 104 (1986) 339-372. DOI:10.1016/0040-6031(86)85208-X |
| [17] |
X. Ye, S. Hui, X. He, et al., J. Mater. Chem. B 2 (2014) 3667-3673. DOI:10.1039/C4TB00202D |
| [18] |
W. Zierkiewicz, M. Michalczyk, D. Bienko, D. Michalska, T. Zeegers-Huyskens, Int. J. Quantum Chem. 117 (2017) e25369.
|
| [19] |
Y. Zhang, A.M. Schnoes, A.R. Clapp, ACS Appl. Mater. Interfaces 2 (2010) 3384-3395. DOI:10.1021/am100996g |
| [20] |
L. Li, J. Liu, X. Yang, et al., Nanotechnology 27 (2016) 105603. DOI:10.1088/0957-4484/27/10/105603 |
| [21] |
S. Kanchi, P. Singh, K. Bisetty, Arab. J. Chem. 7 (2014) 11-25. DOI:10.1016/j.arabjc.2013.04.026 |
| [22] |
N.S. Lawrence, J. Davis, L. Jiang, et al., Electroanalysis 12 (2000) 1453-1460. DOI:10.1002/1521-4109(200012)12:18<1453::AID-ELAN1453>3.0.CO;2-Z |
| [23] |
Y. Zou, L. Zhang, L. Yang, et al., J. Controlled Release 273 (2018) 160-179. DOI:10.1016/j.jconrel.2018.01.023 |
| [24] |
S. Li, S. Dong, W. Xu, et al., Adv. Sci. 5 (2018) 1700527. DOI:10.1002/advs.201700527 |
| [25] |
B. Gemici, J.L. Wallace, Methods Enzymol. 555 (2015) 169-193. DOI:10.1016/bs.mie.2014.11.034 |
| [26] |
H.Y. Chang, M.J. Sheu, C.H. Yang, et al., Evid. Based Compl. Alt. Med. 17 (2011) 9112-9119. DOI:10.1093/ecam/nep027 |
 2020, Vol. 31
2020, Vol. 31 

