b Engineering Research Center of Molecular Medicine of Ministry of Education, Key Laboratory of Fujian Molecular Medicine, Key Laboratory of Xiamen Marine and Gene Drugs, School of Biomedical Sciences, Huaqiao University, Xiamen 361021, China
Substituted benzoquinones are key structural motifs, existingina broad spectrum of biologically active natural products and important pharmaceuticals [1]. Among them, benzoquinone imines are important synthons in organic synthesis, which are also widely used in the dye industry and inhibitors against the photo-oxidation ofpolymers[2].Forexample, early in1983, Rajappagroup reported a novel synthesis of 5-acylaminobenzimidazole-2-carbamates through intramolecular regioselective addition of benzoquinone imines [3]. In 2001, Nair group reported a dipolar cycloaddition of carbonyl ylides to para-benzoquinone imines, which could be used as a facile route to bicyclo[3.2.1] and [2.2.1] systems [4]. After that, Nair group reported a three component reaction involving isocyanides, dimethyl acetylenedicarboxylate and benzoquinone imines as a facile synthesis of spiro-fused γ-iminolactams [5]. In addition, Parker group reported an annulation of enolizable vinyl benzoquinone imines for the synthesis of dihydroquinolines, quinolines and indoles [6]. Recently, catalytic transformation of C–H bonds into valuable C–N bonds offers an efficient synthetic approach to constructN-functionalized molecules [7]. Over the last fewdecades, transition-metal catalysis provesas a powerful tool for the direct C–H bonds amination reactions. For example, in 2008, Gaunt group reported an oxidative Pd(Ⅱ)-catalyzed C–H bond amination to synthesize carbazoles at room temperature [8]. In 2012, White group reported an Iron-catalyzed intramolecular allylic C–H amination to synthesize sulfonamides [9]. Recently, our group has developed a series of Ir-catalyzed direct C–H bond amination of arylquinazolinones [10]. On the other hand, radical C–H bond functionalization has emerged as a promising approach because of their high reactivity with high atom- and step-economy [11]. For example, Studer and co-workers have disclosed effective methods of iron-initiated radical C–H functionalization for the direct synthesis of phenanthridines, fluorenones and xanthones starting from commercially available aromatic aldehydes [12]. For this direction, our group has developed a ferrocene-initiated oxidative cyclization of benzaldehyde with alkyne as a new strategy to substituted indenones [13]. In our continuing effort to demonstrate the clean C–H functionalization [14], herein, we present a new ferrocene-initiated radical reaction of benzoquinone with amines to synthesize diaminobenzoquinone imines (Scheme 1). This protocol features easy operation, low loading of ferrocene (0.5 mol%), cheap and easilyavailable starting materials, high efficiencyand tolerance of a broad range of substrates.

|
Download:
|
| Scheme 1. Ferrocene-initiated radical reaction of benzoquinone with amine. | |
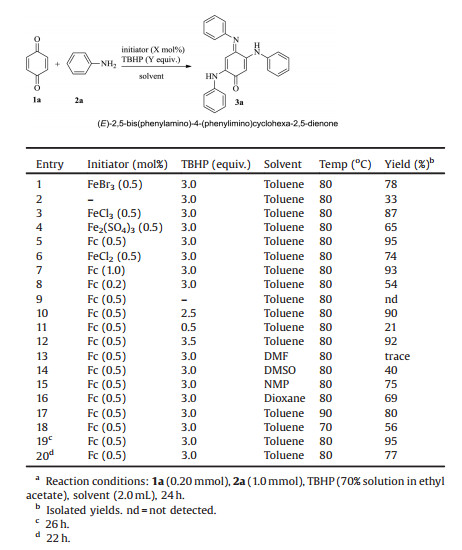
Initially, the reaction of benzoquinone (BQ) (1a) with aniline (2a) was chosen as a model reaction to optimize various reaction conditions (Table 1). The results revealed that (E)-2, 5- bis(phenylamino)-4-(phenylimino)cyclohexa-2, 5-dienone (3a) was obtained as a main product in 78% yield in toluene at 80 ℃ when FeBr3 (0.5 mol%) was used as an initiator and t-BuOOH (TBHP) (3.0 equiv.) was used as the oxidant (Table 1, entry 1). Only 33% yield of the target 3a was achieved when the reaction was carried out in the absence of an initiator (Table 1, entry 1 vs. 2). Iron salts, such as FeCl3, Fe2(SO4)3, FeCl2 and ferrocene (Fc), were screened. Ferrocene gave the highest yield (95%) (Table 1, entry 1 vs. entries 3–6). Better result was achieved with 0.5 mol% of ferrocene (Table 1, entry 5 vs. entries 7–8). Moreover, 3.0 equiv. of TBHP favor this reaction (Table 1, entry 5 vs. entries 9–12). Toluene was demonstrated to be better than other solvents, such as DMF, DMSO, NMP (N-methyl pyrrolidone) and dioxane (Table 1, entry 5 vs. entries 13–16). The yield of 3a decreased when the reaction temperature and reaction time were changed (Table 1, entries 17–20). Based on the results, the optimal reaction conditions were identified as follows: toluene as solvent, at 80 ℃, ferrocene (0.5 mol%) as an initiator and t-BuOOH (TBHP) (3.0 equiv.) as an oxidant (Table 1, entry 5).
|
|
Table 1 Optimization of the reaction conditions.a |
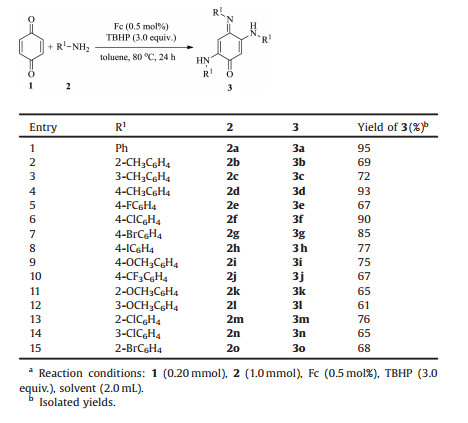
With the optimized reaction conditions in hand, the scope of the substrates was examined (Table 2). Benzoquinone (1a) reacted smoothly with aniline (2a) and its derivatives (2b-1o) to give the desired products (3a-3o) in moderate to good yields (61%–95%). Methyl group at the ortho-, meta-, and para-position of aniline provided the corresponding products 3b-3d in 69%, 72% and 93% yields, which indicated that steric effect of substituted groups slightly affected this transformation. Halogen groups, such as F, Cl, Br and I at the 4-position of aniline provided the corresponding products 3e-3h in 67%, 90%, 85% and 77% yields, respectively. Other groups, such as methoxyl and trifluoromethyl, were well tolerated and gave the corresponding products in satisfactory yields (3i and 3j) (75% and 67%). Compared to the yield of 3d, these results indicated that the electron-withdrawing groups have certain influence on the efficiency of this coupling reaction. Moreover, methoxyl and halogen groups, such as Cl, and Br at the 2- or 3- position of aniline could also provide the corresponding products 3k-3o in 65%, 61%, 76%, 65% and 68% yields, respectively. However, other quinones, aliphatic amines, or amides, such as 2-methyl cyclohexa-2, 5-diene-1, 4-dione, 2-(tert-butyl)cyclohexa-2, 5-diene- 1, 4-dione, propylamine or benzamide, failed to give the desired products.
|
|
Table 2 Scope of substrates.a |
To clarify the reaction mechanism, control experiments were carried out (Scheme 2). First, no 3a was achieved and 72% yield of 4-(phenylamino)phenol (4) was generated in the absence of an initiator and an oxidant(Scheme 2a).Additionally, 4-(phenylamino) phenol(4)could react with 2a to give the expected product 3a in 98% yield under the optimized conditions (Scheme 2b). These results suggested that compound 4 could be a key intermediate in this reaction. When a radical scavenger, TEMPO, was introduced intothe reaction, no target product (3a) was achieved, which suggested that a radical pathway might be involved in this reaction (Scheme 2c).

|
Download:
|
| Scheme 2. Control experiments. | |
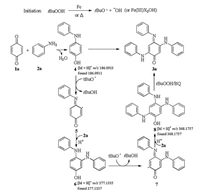
Based on the results obtained, a plausible reaction mechanism is proposed as shown in Scheme 3. First, initiation occurs by reducing t-BuOOH with ferrocene to give the tert-butoxyl radical as well as an Fe(Ⅲ)-complex. The tert-butoxyl radical then abstracts the H-atom from the 4-(phenylamino)phenol (4) generated from 1a and 2a to give a radical intermediate 5, which could reacted with 2a to generate 3, 4-bis(phenylamino)phenol (6). Subsequently, another radical intermediate 7 was generated from the reaction of tert-butoxyl radical with compound 6, which could reacted with 2a to generate 2, 4, 5-tris(phenylamino)phenol (8). Then, the intermediate 8 could be oxidized by TBHP or benzoquinone to the target product 3a. In this reaction, intermediates 4, 6, and 8 were detected by HRMS (Figs. S1-S3 in Supporting information)
|
Download:
|
| Scheme 3. Proposed reaction mechanism. | |
In summary, we have demonstrated a ferrocene-initiated radical reaction of benzoquinone with amines for direct access to diaminobenzoquinone imines, which was initiated by low loading of ferrocene (0.5 mol%) with high yields and a broad substrate scope was tolerated. Reaction mechanism studies have demonstrated the formation of two radical species as key intermediates, which might be responsible for the sequential formation of C–N bonds. Further study on the application of this radical reaction system is ongoing in our laboratory.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21572072), 111 Project (No. BC2018061) and Y. Feng thanks the financial support of Scientific Research Foundation of Xiamen Huaxia University (No. HX201807), Outstanding Youth Scientific Research Cultivation Plan in Fujian Province University (No. 201808).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2019.09.026.
| [1] |
(a) E. Prochazka, B.I. Escher, M.J. Plewa, F.D.L. Leusch, Chem. Res. Toxicol. 28 (2015) 2059-2068; (b) R. Mout, Z.D. Xu, A.K.H. Wolf, V.J. Davisson, G.K. Jarori, Malar. J.11 (2012) 54-54; (c) D. Tasdemir, R. Brun, V. Yardley, S.G. Franzblau, P. Ruedi, Chem. Biodiv. 3 (2006) 1230-1237; (d) L.F. Fieser, E.M. Chamberlin, J. Am. Chem. Soc. 70 (1948) 71-75; (e) B. Joy, S.N. Kumar, M.S. Soumya, et al., Phytomedicine 21 (2014) 1292-1297; (f)A.Cavalli, M.L.Bolognesi, S.Capsoni, etal., Angew.Chem.Int.Ed.46 (2007)3689-3692; (g) T.J. Monks, P. Hanzlik, G.M. Cohen, D. Ross, D.G. Graham, Toxicol. Appl. Pharmacol. 112 (1992) 2-16. |
| [2] |
(a) J. Yu, H. Zhang, Q. Lu, et al., Chem. Ind. Eng. Progress 34 (2015) 1115-1121; (b) M.R. Halhalli, B. Sellergren, Polym. Chem. 6 (2015) 7320-7332; (c) M.A. Hanna, M.M. Girges, Acta Polym. 41 (1990) 354-360; (d) S. Rajappa, S.J. Shenoy, Tetrahedron 42 (1986) 5739-5746. |
| [3] |
S. Rajappa, R. Sreenivasan, A.V. Rane, Tetrahedron Lett. 24 (1983) 3155-3158. DOI:10.1016/S0040-4039(00)88121-6 |
| [4] |
V. Nair, C. Rajesh, R. Dhanya, A.U. Vinod, Tetrahedron Lett. 42 (2001) 2045-2046. DOI:10.1016/S0040-4039(01)00072-7 |
| [5] |
V. Nair, R. Dhanya, S. Viji, Tetrahedron 61 (2005) 5843-5848. DOI:10.1016/j.tet.2005.04.008 |
| [6] |
K.A. Parker, T.L. Mindt, Org. Lett. 4 (2002) 4265-4268. DOI:10.1021/ol026849x |
| [7] |
(a) Y. Park, Y. Kim, S. Chang, Chem. Rev. 117 (2017) 9247-9301; (b) J. Kim, S. Chang, Angew. Chem. Int. Ed. 53 (2014) 2203-2207; (c) T. Kang, Y. Kim, D. Lee, Z. Wang, S. Chang, J. Am. Chem. Soc.136 (2014) 4141-4144; (d) H. Hwang, J. Kim, J. Jeong, S. Chang, J. Am. Chem. Soc. 136 (2014) 10770-10776; (e) C. Pi, X. Cui, Y. Wu, J. Org. Chem. 80 (2015) 7333-7339; (f) M.E. Wei, L.H. Wang, Y.Y. Li, X. Cui, Chin. Chem. Lett. 26 (2015) 1336-1340; (g) X. Han, P. Lin, Q. Li, Chin. Chem. Lett. 30 (2019) 1495-1502; (h) S. Yuan, S. Wang, M. Zhao, et al., Chin. Chem. Lett. 31 (2020) 349-352; (i) Q. Huang, L. Zhu, D. Yi, X. Zhao, W. Wei, Chin. Chem. Lett. 31 (2020) 373-376; (j) X. Zhang, S. Dong, Q. Ding, X. Fan, G. Zhang, Chin.Chem. Lett. 30 (2019) 375-378; (k) L. Xie, S. Peng, L. Jiang, et al., Org. Chem. Front. 6 (2019) 167-171; (l) L. Xie, S. Peng, F. Liu, et al., ACS Sustainable Chem. Eng. 7 (2019) 7193-7199. |
| [8] |
J.A. Jordan-Hore, C.C.C. Johansson, M. Gulias, E.M. Beck, M.J. Gaunt, J. Am. Chem. Soc. 130 (2008) 16184-16186. DOI:10.1021/ja806543s |
| [9] |
S.M. Paradine, M.C. White, J. Am. Chem. Soc. 134 (2012) 2036-2039. DOI:10.1021/ja211600g |
| [10] |
(a) Y. Feng, Y. Li, Y. Yu, L. Wang, X. Cui, RSC Adv. 8 (2018) 8450-8454; (b) Y. Feng, Z. Zhang, Q. Fu, et al., Chin. Chem. Lett. 31 (2020) 58-60. |
| [11] |
(a) C. Liu, D. Liu, A. Lei, Acc. Chem. Res. 47 (2014) 3459-3470; (b) S.A. Girard, T. Knauber, C.J. Li, Angew. Chem. Int. Ed. 53 (2014) 74-100; (c) R. Braslau, M.O. Anderson, F. Rivera, et al., Tetrahedron 58 (2002) 5513-5523; (d) S. Bath, N.M. Laso, H. Lopez-Ruiz, B. Quiclet-Sire, S.Z. Zard, Chem. Commun. 34 (2003) 204-205; (e) J. Wang, C. Liu, J. Yuan, A. Lei, Angew. Chem. Int. Ed. 52 (2013) 2256-2259; (f) J. Xie, J. Yu, M. Rudolph, F. Rominger, A.S. Hashmi, Angew. Chem. Int. Ed. 55 (2016) 9416-9421; (g) C. Wang, J. Qin, X. Shen, et al., Angew. Chem. Int. Ed. 55 (2016) 685-688; (h) L.Y. Xie, S. Peng, F. Liu, et al., Org. Chem. Front. 5 (2018) 2604-2609; (i) L.Y. Xie, S. Peng, F. Liu, et al., Adv. Synth. Catal. 360 (2018) 4259-4264; (j) L. Xie, T. Fang, J. Tan, et al., Green Chem. 21 (2019) 3858-3863. |
| [12] |
(a) D. Leifert, C.G. Daniliuc, A. Studer, Org. Lett. 15 (2013) 6286-6289; (b) S. Wertz, D. Leifert, A. Studer, Org. Lett. 15 (2013) 928-931. |
| [13] |
Y. Feng, H. Zhang, Y. Yu, L. Yang, X. Cui, Eur. J. Org. Chem. 16 (2019) 2740-2744. |
| [14] |
(a) L. Wang, D. Xiong, L. Jie, C. Yu, X. Cui, Chin. Chem. Lett. 29 (2018) 907-910; (b) L. Xu, T. Li, L. Wang, X. Cui, J. Org. Chem. 84 (2019) 560-567; (c) Z. Yang, L. Jie, Z. Yao, et al., Adv. Catal. Synth. 1 (2019) 214-258; (d) P. Chao, X. Yin, X. Cui, Y. Ma, Y. Wu, Org. Lett. 7 (2019) 2081-2084; (e) J. Ren, C. Pi, Y. Wu, X. Cui, Org. Lett. 11 (2019) 4067-4071; f) S. Huang, H. Li, X. Sun, et al., Org. Lett. 21 (2019) 5570-5574; (g) B. Wu, Z. Yang, H. Zhang, L. Wang, Cui X, Chem. Commun. 55 (2019) 4190-4193; (h) Z. Yang, Z. Song, L. Jie, L. Wang, X. Cui, Chem. Commun. 55 (2019) 6094-6097; (i) T. Yuan, C. Pi, C. You, et al., Chem. Commun. 55 (2019) 163-166; (k) T. Wan, S. Du, C. Pi, Y. Wang, R. Li, Y. Wu, X. Cui, ChemCatChem 11 (2019) 3791-3796; (l) S. Du, C. Pi, T. Wan, Y. Wu, X. Cui, Adv. Synth. Catal. 361 (2019) 1766-1770; (m) Z.H. Shen, C. Pi, X. Cui, Y. Wu, Chin. Chem. Lett. 30 (2019) 1374-1378. |
 2020, Vol. 31
2020, Vol. 31