b Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China;
c Shanghai Collaborative Innovation Center for Biomanufacturing Technology, Shanghai 200237, China
Chiral polycyclic indoles are privileged structures found in a great many of natural bioactive products and drug molecules [1]. Among them, chiral pyrrolo[1, 2-a]indole scaffold has received much attentions owing to the remarkable biological activities [2], and development of the straightforward approaches to such structures has been an important task [3-6]. Among the known strategies, catalytic asymmetric [3 + 2] annulations were considered to be the most efficient and economical approach [6]. In 2013, Enders and coworkers reported an elegant N-heterocyclic carbene catalyzed asymmetric [3 + 2] annulation of 2-nitrovinylindoles with α-chloroaldehydes (Scheme 1, Eq. 1) [6a]. Recently, α- ketoesters and hemiacetals were successfully applied in the catalytic asymmetric [3 + 2] annulations with 2-nitrovinylindoles by our (Scheme 1, Eq. 2) [6b] and Liu's group (Scheme 1, Eq. 3) [6c]. Besides, the research group of Schneider [6d-e], Rodríguez [6f], and Shi [6g] independently developed the Brønsted acid catalyzed asymmetric [3 + 2] annulations of 2-indolyl compounds with electron-rich alkenes for the direct synthesis of structurally diverse pyrrolo[1, 2-a]indoles. Despite these remarkable advances, the construction of chiral pyrrolo[1, 2-a]indoles bearing a quaternary stereocenter at C2 position still challenging and highly desirable remains (Scheme 2).
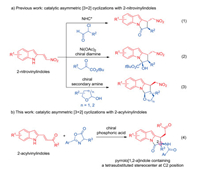
|
Download:
|
| Scheme 1. Catalytic asymmetric [3 + 2] annulations with 2-nitrovinylindoles and 2-acylvinylindoles. | |
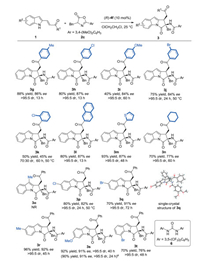
|
Download:
|
| Scheme 2. Substrate scope of 2-acylvinylindoles 1. Reactions were performed with 1 (0.1 mmol), 2c (0.15 mmol) in 1.0 mL 1, 2-dichloroethane at 25 ℃. Isolated yields of two diastereomers; dr values were determined by 1H NMR spectroscopy; ee values were determined by chiral HPLC analysis. aThe gram-scale reaction was performed, affording product 3s with 1.06 g. | |
Azlactones appear as a class of useful reactants in organic synthesis because they are readily available and versatile reactive character [7]. In particular, the catalytic asymmetric [3 + 2] [8] and [4 + 2] [9] annulations with azlactones have been well-documented for the synthesis of diverse cyclic structures. In conjunction with our continuing efforts in the diversity-oriented synthesis of chiral polycyclic indoles [6b, 10], we envisaged that azlactones could serve as a two-atom building block, engage electronwithdrawing group substituted 2-vinylindoles via a tandem Michael/ring open process, thus delivering chiral pyrrolo[1, 2-a] indoles with an aza-quaternary stereocenter at C2 position by formal [3 + 2] annulation. To achieve this goal, we reported herein chiral phosphoric acid catalyzed asymmetric [3 + 2] annulation of 2-acylvinylindoles with azlactones, providing a straightforward approach to structurally diverse pyrrolo[1, 2-a]indoles in good to high diastereo- and enantioselectivities (Scheme 1, Eq. 4).
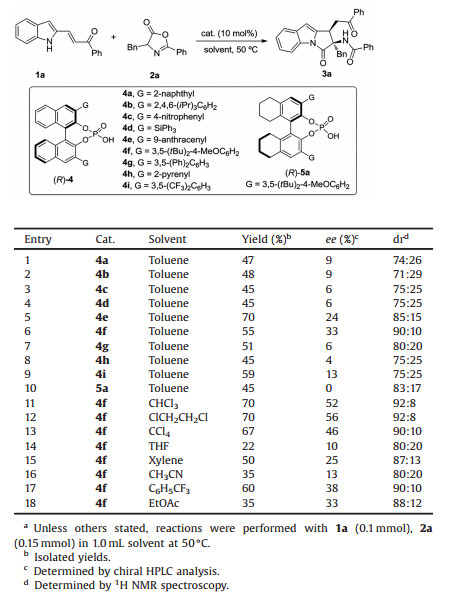
Initially, β-(indol-2-yl)-α, β-unsaturated ketone 1a and azlactone 2a were chosen as model substrates to test the feasibility of this [3 + 2] annulation by employing chiral phosphoric acid [11] as catalyst (Table 1). A series of BINOL-derived chiral phosphoric acids 4 were evaluated for this [3 + 2] annulation in toluene at 50 ℃ (entries 1–9), and the desired [3 + 2] annulation proceeded smoothly. Cycloadduct 3a was generated in 55% yield, 33% ee and 90:10 dr when using chiral phosphoric acid 4f as catalyst (entry 6). Subsequently, the backbone of 4f was changed from BINOL to H8-BINOL to improve the stereoselectivity (entry 10). Unfortunately, only racemic product was observed with chiral phosphoric acid 5a. To further improve the enantioselectivity, the solvent effect in this reaction was investigated (entries 11–18), and the chlorinated solvents proffer better results (entries 11–13), with 1, 2-dichloroethane as the best choice (entry 12, 70% yield, 56% ee, 92:8 dr).
|
|
Table 1 Optimization of reaction conditions.a |
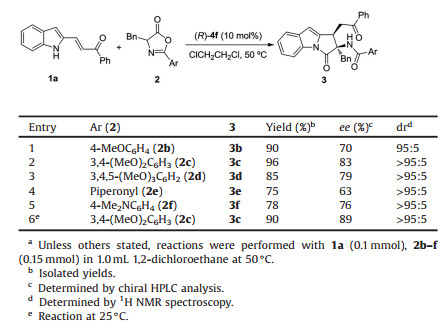
Having established the optimal catalyst and solvent, we next turned our attention to improve the enantioselectivity by modifying the substituent Ar on azlactone 2 (Table 2). Pleasingly, when the electron-donating group OMe was introduced on the benzene ring, an obvious improved diastereo- and enantioselectivity was detected (entries 1–3). In particular, the azlactone 2c with 3, 4-dimethoxyphenyl group provided the corresponding pyrrolo[1, 2-a]indole 3c in 96% yield with 83% ee and >95:5 dr (entry 2). Next, the other electron-donating group was studied, such as piperonyl and 4-dimethylaminophenyl, unfortunately, no better result was gained (entries 4, 5). Subsequently, the reaction temperature was cooled to room temperature to increase the enantioselectivity, and the ee value of product 3c was raised to 89% (entry 6).
|
|
Table 2 Evaluation of the substituent Ar on azlactone.a |
After identifying the optimal reaction conditions and the suitable substituent on the azlactone, the substrate scope of 2- acylvinylindoles 1 was evaluated. A wide range of β-(indol-2-yl)-α, β-unsaturated ketone 1 bearing electron-deficient groups and electron-rich groups on the benzene ring proceeded smoothly, affording corresponding [3 + 2] cycloadducts 3g–j in good yield with high diastereoselectivities (>95:5 dr) and good enantioselectivities (84%–87% ee), except the ortho-substituent on the phenyl ring, which provided diminished stereoselectivity (70:30 dr, 45% ee). Additionally, 2-naphthyl and 2-thienyl substituted 2- indolylpropenone were also tolerated in this reaction and delivered the corresponding products 3l and 3m with excellent diastereoselectivities (>95:5 dr) and good enantioselectivities (87% ee). The reaction with alkyl-substituted β-(indol-2-yl)-α, β- unsaturated ketone still proceeded smoothly, however, lower enantioselectivity was observed (77% ee). Subsequently, the substituent on indole ring was studied. The reaction was dull when the methyl group was introduced on the C3 position of indole. β-(Indol-2-yl)-α, β-unsaturated ketone bearing various substituents on C5 - C6 positions of indole were successfully applied in the reaction, and pyrrolo[1, 2-a]indoles 3p-3s were generated in high yields (70%-96%) with good enantioselectivities (82%-92% ee). Notably, β-(Indol-2-yl)-α, β-unsaturated ketone with Br atom at C7 position only provided the Michael adduct under the standard conditions. Inspired by Scheidt's report [12], cooperative catalyst urea 6 (10 mol%) was added to promote the annulation process, pleasingly, the cycloadduct 3t was gained in 70% yield, albeit with a decreased enantioselectivity (76% ee). In addition, the relative and absolute configurations of product 3q were unambiguously confirmed by single-crystal X-ray analysis (crystallographic data with CCDC No. 1950068 is deposited in Cambridge Centre. Moreover, a gram-scale experiment was conducted, affording product 3s (1.06 g) in 90% yield, 91% ee, and >95:5 dr.
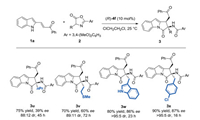
Next we evaluated the substrate scope of azlactone 2 (Scheme 3). The benzyl group was crucial for the enantioselectivity controlling. Azlactones derived from norvaline and methionine provided the corresponding products 3u and 3v in good yields (70%–75%) with a decreased diastereo- and enantioselectivities (39%–60% ee, 88:12–89:11 dr). Azlactones bearing 3-indolyl and 4- chlorophenyl were also amenable to this transformation, furnishing products 3w and 3x in good yields (80%–90%) and good enantioselectivities (86%–87% ee).
|
Download:
|
| Scheme 3. Substrate scope of azlactones 2. Reactions were performed with 1a (0.1 mmol), 2 (0.15 mmol) in 1.0 mL 1, 2-dichloroethane at 25 ℃. Isolated yields of two diastereomers; dr values were determined by 1H NMR spectroscopy; ee values were determined by chiral HPLC analysis. | |
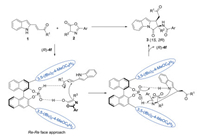
We proposed a stereocontrolled model to explicate the observed stereochemical preference. As illustrated in Scheme 4, chiral phosphoric acid (R)-4f simultaneously activated the two substrates by hydrogen-bonding interactions, and the Re face of alkene was attacked by the Re face of the enolate of azlactone. Subsequently, the NH on indole ring and the imine of azlactone were activated via the hydrogen-bonding interactions with (R)-4f respectively, which facilitated the intramolecular aminolysis, and afforded the product (1S, 2R)-3.
|
Download:
|
| Scheme 4. Proposed transition states. | |
In conclusion, we have developed a practical synthetic route to polysubstituted pyrrolo[1, 2-a]indoles via organocatalytic asymmetric [3 + 2] annulation of substituted 2-vinylindoles with azlactones. By using BINOL-derived chiral phosphoric acid (R)-4f as a catalyst under mild conditions, a series of pyrrolo[1, 2-a] indoles bearing a tetrasubstituted stereocenter at C2 position were obtained in good yields and good diastereo- and enantioselectivities. Further studies about the annulation of 2-vinylindoles for the synthesis of polycyclic indoles are currently underway.
AcknowledgmentsThis work is supported by the Fundamental Research Funds for the Central Universities, the China Postdoctoral Science Foundation (Nos. 2018M632037, 2019T120310) and Shanghai Sailing Program (No. 18YF140560).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.008.
| [1] |
(a) G.R. Humphrey, J.T. Kuethe, Chem. Rev. 106 (2006) 2875-2911; (b) M. Inman, C.J. Moody, Chem. Sci. 4 (2013) 29-41; (d) R. Dalpozzo, Chem. Soc. Rev. 44 (2015) 742-778; (c) S. Lancianesi, A. Palmieri, M. Petrini, Chem. Rev. 114 (2014) 7108-7149. |
| [2] |
(a) L.S. Fernandez, M.S. Buchanan, A.R. Carroll, et al., Org. Lett. 11 (2009) 329-332; (b) I.S. Marcos, R.F. Moro, I.Costales, P.Basabe, D. Díez, Nat.Prod.Rep. 30 (2013) 1509-1526; (c) E.O.M. Orlemans, W. Verboom, M.W. Scheltinga, et al., J. Med. Chem. 32 (1989) 1612-1620; (d) U. Galm, M.H. Hager, S.G.V. Lanen, et al., Chem. Rev. 105 (2005) 739-758. |
| [3] |
(a) R.M. Wilson, R.K. Thalji, R.G. Bergman, J.A. Ellman, Org. Lett. 8 (2006) 1745-1747; (b) Q. Cai, C. Zheng, S.L. You, Angew. Chem. Int. Ed. 49 (2010) 8666-8669; (c) A. Ghosh, L.M. Stanley, Chem. Commun. 50 (2014) 2765-2768; (d) T. Shibata, N. Ryu, H. Takano, Adv. Synth. Catal. 357 (2015) 1131-1135; (e) D. Janssen-Müller, M. Schedler, M. Fleige, C.G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 54 (2015) 12492-12496. |
| [4] |
A. Ghosh, J.A. Walker, A. Ellern, L.M. Stanley, ACS Catal. 6 (2016) 2673-2680. DOI:10.1021/acscatal.6b00365 |
| [5] |
(a) L. Hong, W. Sun, C. Liu, L. Wang, R. Wang, Chem. -Eur. J.16 (2010) 440-444; (b) M. Zeng, W. Zhang, S.L. You, Chin. J. Chem. 30 (2012) 2615-2623; (c) H.G. Cheng, L.Q. Lu, T. Wang, et al., Angew. Chem. Int. Ed. 52 (2013) 3250-3254; (d) Y. Zhang, X. Liu, X. Zhao, et al., Chem. Commun. 49 (2013) 11311-11313; (e) H. Lu, J.B. Lin, J.Y. Liu, P.F. Xu, Chem. -Eur. J. 20 (2014) 11659-11663; (f) H.L. Ma, J.Q. Li, L. Sun, et al., Tetrahedron 71 (2015) 3625-3631; (g) Y.J. Yang, Y. Ji, L. Qi, G. Wang, X.P. Hui, Org. Lett. 19 (2017) 3271-3274; (h) N.K. Li, J.Q. Zhang, B.B. Sun, H.Y. Li, X.W. Wang, Org. Lett. 19 (2017) 1954-1957. |
| [6] |
(a) Q. Ni, H. Zhang, A. Grossmann, et al., Angew. Chem. Int. Ed. 52 (2013) 13562-13566; (b) W.L. Yang, Z.T. Sun, H. Sun, W.P. Deng, Chin. J. Chem. 37 (2019) 216-220; (c) C.C. Xie, R. Tan, Y.K. Liu, Org. Chem. Front. 6 (2019) 919-924; (d) K. Bera, C. Schneider, Chem. -Eur. J. 22 (2016) 7074-7078; (e) K. Bera, C. Schneider, Org. Lett. 18 (2016) 5660-5663; (f) A. Galván, A.B. González-Pérez, R. Álvarez, et al., Angew. Chem. Int. Ed. 55 (2016) 3428-3432; (g) Z.Q. Zhu, L. Yin, Y. Wang, et al., Org. Chem. Front. 4 (2017) 57-68. |
| [7] |
(a) R.A. Mosey, J.S. Fisk, J.J. Tepe, Tetrahedron Asymmetry 19 (2008) 2755-2762; (b) J.S. Fisk, R.A. Mosey, J.J. Tepe, Chem. Soc. Rev. 36 (2007) 1432-1440. |
| [8] |
(a) S. Dong, X. Liu, Y. Zhu, et al., J. Am. Chem. Soc. 135 (2013) 10026-10029; (b) G. Li, W. Sun, J. Li, et al., Chem. Commun. 51 (2015) 11280-11282; (c) X. Liu, Y. Wang, D. Yang, et al., Angew. Chem. Int. Ed. 55 (2016) 8100-8103; (d) C. Ma, J.Y. Zhou, Y.Z. Zhang, G.J. Mei, F. Shi, Angew. Chem. Int. Ed. 57 (2018) 5398-5402; (e) J.C. Yu, L.M. Yu, X.Y. Zhao, et al., Org. Chem. Front. 5 (2018) 2040-2044; (f) L. Xie, S. Dong, Q. Zhang, X. Feng, X. Liu, Chem. Commun. 55 (2019) 87-90. |
| [9] |
(a) J. Jiang, J. Qing, L.Z. Gong, Chem. -Eur. J. 15 (2009) 7031-7034; (b) S. Dong, X. Liu, X. Chen, et al., J. Am. Chem. Soc. 132 (2010) 10650-10651; (c) S. Dong, X. Liu, Y. Zhang, L. Lin, X. Feng, Org. Lett. 13 (2010) 5060-5063; (d) H. Hu, Y. Liu, J. Guo, et al., Chem. Commun. 51 (2015) 3835-3837; (e) J. Hejmanowska, A. Albrecht, J. Pieţa, Ł. Albrecht, Adv. Synth. Catal. 357 (2015) 3843-3848; (f) S.Y. Zhang, M. Lv, S.J. Yin, et al., Adv. Synth. Catal. 358 (2016) 143-153; (g) Y.C. Zhang, Q.N. Zhu, X. Yang, L.J. Zhou, F. Shi, J. Org. Chem. 81 (2016) 1681-1688; (h) J.R. Chen, Q. Wei, H.G. Liu, Z.C. Cheng, W.J. Xiao, Chem. -Eur. J. 22 (2016) 6774-6778; (i) J. Zhou, M.L. Wang, X. Gao, G.F. Jiang, Y.G. Zhou, Chem. Commun. 53 (2017) 3531-3534; (j) L. Zhang, Y. Liu, K. Liu, et al., Org. Biomol. Chem. 15 (2017) 8743-8747; (k) S. Ruan, X. Lin, L. Xie, et al., Org. Chem. Front. 5 (2018) 32-35; (l) A.K. Simlandy, B. Ghosh, S. Mukherjee, Org. Lett. 21 (2019) 3361-3366. |
| [10] |
(a) W.L. Yang, C.Y. Li, W.J. Qin, et al., ACS Catal. 6 (2016) 5685-5690; (b) Y.Z. Liu, S.J. Shang, J.Y. Zhu, W.L. Yang, W.P. Deng, Adv. Synth. Catal. 360 (2018) 2191-2203; (c) X. Zheng, W.L. Yang, Y.Z. Liu, S.X. Wu, W.P. Deng, Adv. Synth. Catal. 360 (2018) 2843-2853. |
| [11] |
(a) T. Akiyama, Chem. Rev. 107 (2007) 5744-5758; (b) M. Terada, Synthesis (2010) 1929-1982; (c)A.Zamfir, S. Schenker, M. Freund, S.B. Tsogoeva, Org.Biomol. Chem. 8 (2010) 5262-5276; (d) M. Rueping, A. Kuenkel, I. Atodiresei, Chem. Soc. Rev. 40 (2011) 4539-4549; (e) D. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 114 (2014) 9047-9153; (f) T. James, M. van Gemmeren, B. List, Chem. Rev. 115 (2015) 9388-9409. |
| [12] |
M.A. Maskeri, M.J. O'Connor, A.A. Jaworski, A.V. Davies, K.A. Scheidt, Angew. Chem. Int. Ed. 57 (2018) 17225-17229. DOI:10.1002/anie.201811383 |
 2020, Vol. 31
2020, Vol. 31